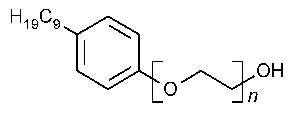
Nonoxynol 9
»Nonoxynol 9is an anhydrous liquid mixture consisting chiefly of monononylphenyl ethers of polyethylene glycols corresponding to the formula:
C9H19C6H4(OCH2CH2)nOH,
in which the average value of nis about 9.It contains not less than 90.0percent and not more than 110.0percent of nonoxynol 9.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
Its IRabsorption spectrum,obtained by spreading a capillary film of it between sodium chloride plates,exhibits maxima at 1117cm-1(strong);at 1512,1582,and 1610cm-1(medium,sharp);at 2871,2928,and 2956cm-1(strong,unresolved);at 831cm-1(medium,broad);and at 1250cm-1(medium,sharp).
B:
The retention time of the major peak in the chromatogram of the Assay preparationcorresponds to that in the chromatogram of the Standard preparationas obtained in the Assay.
Cloud point—
Transfer 1.0g to a 250-mLbeaker,add 99g of water,and mix to dissolve.Pour about 30mLof the solution into a 70-mLtest tube.Support the test tube in a hot water bath,and stir the contents with a thermometer constantly until the solution becomes cloudy,then remove the test tube from the bath immediately,so that the temperature rises not more than 2 further,and continue stirring.The cloud point is the temperature at which the solution becomes sufficiently clear that the entire thermometer bulb is seen plainly:it is between 52
further,and continue stirring.The cloud point is the temperature at which the solution becomes sufficiently clear that the entire thermometer bulb is seen plainly:it is between 52 and 56
and 56 .
.
Acid value á401ñ:
not more than 0.2.
Water,Method Iá921ñ:
not more than 0.5%.
Polyethylene glycol—
Transfer about 10g,accurately weighed,to a 250-mLbeaker.Add 100mLof ethyl acetate,and stir on a magnetic stirrer to effect solution.Transfer,with the aid of 100mLof 5Nsodium chloride,to a pear-shaped,500-mLseparator fitted with a glass stopper.Insert the stopper,and shake vigorously for 1minute.Remove the stopper carefully to release the pressure.Immerse a thermometer in the mixture,and support the separator so that it is partially immersed in a water bath maintained at 50 .Swirl the separator gently while letting the internal temperature rise to between 40
.Swirl the separator gently while letting the internal temperature rise to between 40 and 45
and 45 ,then immediately remove the separator from the bath,dry the outside surface,and drain the salt (lower)layer into another pear-shaped,500-mLseparator.In the same manner,extract the ethyl acetate layer a second time with 100mLof fresh 5Nsodium chloride,combining the two aqueous extracts.Discard the ethyl acetate layer.Wash the combined aqueous layers with 100mLof ethyl acetate,using the same technique,and drain the salt (lower)layer into a clean pear-shaped,500-mLseparator.Discard the ethyl acetate layer.Extract the aqueous layer with two successive 100-mLportions of chloroform,draining the chloroform (lower)layers through Whatman folded filter paper 2V,and combining them into a 250-mLbeaker.Evaporate on a steam bath to dryness,and continue heating until the odor of chloroform is no longer perceptible.Allow the beaker to cool.Add 25mLof acetone,and dissolve the residue on a magnetic stirrer.Filter through Whatman folded filter paper 2Vinto a tared 250-mLbeaker,rinsing with two 25-mLportions of acetone.Evaporate on a steam bath to dryness.Dry in vacuum at 60
,then immediately remove the separator from the bath,dry the outside surface,and drain the salt (lower)layer into another pear-shaped,500-mLseparator.In the same manner,extract the ethyl acetate layer a second time with 100mLof fresh 5Nsodium chloride,combining the two aqueous extracts.Discard the ethyl acetate layer.Wash the combined aqueous layers with 100mLof ethyl acetate,using the same technique,and drain the salt (lower)layer into a clean pear-shaped,500-mLseparator.Discard the ethyl acetate layer.Extract the aqueous layer with two successive 100-mLportions of chloroform,draining the chloroform (lower)layers through Whatman folded filter paper 2V,and combining them into a 250-mLbeaker.Evaporate on a steam bath to dryness,and continue heating until the odor of chloroform is no longer perceptible.Allow the beaker to cool.Add 25mLof acetone,and dissolve the residue on a magnetic stirrer.Filter through Whatman folded filter paper 2Vinto a tared 250-mLbeaker,rinsing with two 25-mLportions of acetone.Evaporate on a steam bath to dryness.Dry in vacuum at 60 for 1hour.Allow the beaker to cool,and weigh:not more than 1.0%of polyethylene glycol is found.
for 1hour.Allow the beaker to cool,and weigh:not more than 1.0%of polyethylene glycol is found.
Free ethylene oxide—
Stripped nonoxynol 9—
Maintain Nonoxynol 9at a temperature of 150 with constant stirring in an open vessel until it no longer displays a peak for ethylene oxide when chromatographed as directed below.
with constant stirring in an open vessel until it no longer displays a peak for ethylene oxide when chromatographed as directed below.
Standard solutions—
[NOTE—Ethylene oxide is toxic and flammable.Prepare these solutions in a well-ventilated hood,using great care.]Chill all apparatus and reagents used in the preparation of standards in a refrigerator or freezer before use.Fill a chilled pressure bottle with liquid ethylene oxide from a lecture bottle,and store in a freezer when not in use.Use a small piece of polyethylene film to protect the liquid from contact with the rubber gasket.Transfer about 100mLof chilled isopropyl alcohol to a 500-mLvolumetric flask.Using a chilled graduated cylinder,transfer 25mLof ethylene oxide to the isopropyl alcohol,and swirl gently to mix.Dilute with additional chilled isopropyl alcohol to volume,replace the stopper,and swirl gently to mix.This stock solution contains about 43.6mg of ethylene oxide per mL.Pipet 25mLof 0.5Nalcoholic hydrochloric acid,prepared by mixing 45mLof hydrochloric acid with 1liter of alcohol,into a 500-mLconical flask containing 40g of magnesium chloride hexahydrate.Shake the mixture to effect saturation.Pipet 10mLof the ethylene oxide solution into the flask,and add 20drops of bromocresol green TS.If the solution is not yellow (acid),add an additional volume,accurately measured,of 0.5Nalcoholic hydrochloric acid to give an excess of about 10mL.Record the total volume of 0.5Nalcoholic hydrochloric acid added.Insert the stopper in the flask,and allow to stand for 30minutes.Titrate the excess acid with 0.5Nalcoholic potassium hydroxide VS.Perform a blank titration,using 10.0mLof isopropyl alcohol instead of ethylene oxide solution,adding the same total volume of 0.5Nalcoholic hydrochloric acid,and note the difference in volumes required.Each mLof the difference in volumes of 0.5Nalcoholic potassium hydroxide consumed is equivalent to 22.02mg of ethylene oxide.Calculate the concentration,in mg per mL,of ethylene oxide in the stock solution.Standardize daily.Store in a refrigerator.Prepare a 1000-ppm standard by pipeting the calculated volume (about 2mL)of cold stock solution which,on the basis of the standardization,contains 88.6mg of ethylene oxide,into a container and adding 87.0g of Stripped nonoxynol 9.Prepare 10-,5-,and 0.5-ppm standards by quantitatively diluting the 1000-ppm standard with additional Stripped nonoxynol 9.
Standard preparations—
Transfer 5±0.01g of each Standard solutionto suitable serum vials equipped with pressure-tight septum closures designed to relieve any excessive pressure,and seal them.
Test preparation—
Transfer 5±0.01g of Nonoxynol 9to a serum vial of the same kind as the vials used for the Standard preparations.
Chromatographic system
(see Chromatography á621ñ)—Use a gas chromatograph equipped with a flame-ionization detector.Under typical conditions,the instrument contains a 6.4-m ×2.1-mm (ID)nickel column packed with 60-to 80-mesh support S9,the column is maintained at 100 ,the injection port is maintained at 160
,the injection port is maintained at 160 ,the detector is maintained at 200
,the detector is maintained at 200 ,and helium is used as the carrier gas at a flow rate of 30mLper minute.The resolution,R,of ethylene oxide and acetaldehyde,upon chromatographing a solution containing 10µg per mLof each in Stripped nonoxynol 9,is not less than 1.5.None of the points used for constructing the straight line Calibrationcurve deviates from the line by more than 10%.
,and helium is used as the carrier gas at a flow rate of 30mLper minute.The resolution,R,of ethylene oxide and acetaldehyde,upon chromatographing a solution containing 10µg per mLof each in Stripped nonoxynol 9,is not less than 1.5.None of the points used for constructing the straight line Calibrationcurve deviates from the line by more than 10%.
Calibration—
Place the vial containing the 10-ppm ethylene oxide Standard preparationin an oven,and heat at 90 for 30minutes.Remove the vial from the oven.Using a gas-tight syringe,immediately inject a 100-µLaliquot of the headspace gas into the gas chromatograph.Obtain the area for the ethylene oxide peak (retention time approximately 8minutes).Raise the temperature of the column to 200
for 30minutes.Remove the vial from the oven.Using a gas-tight syringe,immediately inject a 100-µLaliquot of the headspace gas into the gas chromatograph.Obtain the area for the ethylene oxide peak (retention time approximately 8minutes).Raise the temperature of the column to 200 after ethylene oxide elutes to volatilize heavy components.Re-equilibrate the column at 100
after ethylene oxide elutes to volatilize heavy components.Re-equilibrate the column at 100 .Repeat the foregoing steps,using the vials containing the 5-and 0.5-ppm Standard preparation.Plot area units versus ppm ethylene oxide for the standards on linear graph paper,and draw the best straight line through the points.
.Repeat the foregoing steps,using the vials containing the 5-and 0.5-ppm Standard preparation.Plot area units versus ppm ethylene oxide for the standards on linear graph paper,and draw the best straight line through the points.
Procedure—
Place the vial containing the Test preparationin an oven,and heat at 90 for 30minutes.Remove the vial from the oven.Immediately inject a 100-µLaliquot of the headspace gas into the gas chromatograph,and obtain the area for the ethylene oxide peak.Calculate the concentration of ethylene oxide in the test specimen,in ppm,by the formula:
for 30minutes.Remove the vial from the oven.Immediately inject a 100-µLaliquot of the headspace gas into the gas chromatograph,and obtain the area for the ethylene oxide peak.Calculate the concentration of ethylene oxide in the test specimen,in ppm,by the formula:
rUS,
in which rUis the peak area obtained from the Test preparation,and Sis the slope of the standard curve,in ppm per area unit.Not more than 1ppm is found.
Limit of dioxane—
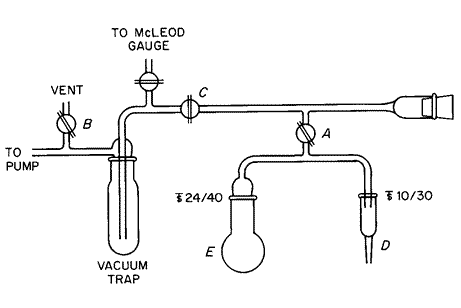
Apparatus—
Assemble a closed-system vacuum distillation apparatus,employing glass vacuum stopcocks (A,B,and C),as shown in the accompanying diagram.The concentrator tube (D)*is made of borosilicate or quartz (not flint)glass,graduated precisely enough to measure the 0.9mLor more of distillate collected and marked so that the analyst can dilute accurately to 2.0mL.
Standard solution—
Prepare a solution of dioxane in water having a known concentration of about 100µg per mL.Use a freshly prepared solution.
Test solution—
Transfer 20.0g to a 50-mLround-bottom flask (E)having a 24/40ground-glass neck joint.Add 1.0mLof water.Place a small polytef-covered stirring bar in the flask,insert the stopper,and stir to mix.Immerse the flask in an ice bath,and chill for about 1minute.Wrap heating tape around the tube connecting the concentrator tube (D)and the round-bottom flask,and apply about 10Vto the tape.Apply a light coating of high-vacuum silicone grease to the ground-glass joints,and connect the concentrator tube to the 10/30joint and the round-bottom flask to the 24/40joint.Immerse the vacuum trap in a Dewar flask filled with liquid nitrogen,close stopcocks Aand B,open stopcock C,and begin evacuating the system with a vacuum pump.Prepare a slurry bath from powdered dry ice and methanol,and raise the bath to the neck of the round-bottom flask.After freezing the contents of the flask for about 10minutes,and when the vacuum system is operating at 0.05-mm pressure or lower,open stopcock Afor 20seconds,then close it.Remove the slurry bath,and allow the flask to warm in air for about 1minute.Immerse the flask in a water bath maintained at a temperature of between 20 and 25
and 25 ,and after about 5minutes warm the water bath to between 35
,and after about 5minutes warm the water bath to between 35 and 40
and 40 (sufficient to liquefy most specimens)while stirring slowly but constantly with the magnetic bar.Cool the water in the bath by adding ice,and chill for about 2minutes.Replace the water bath with the slurry bath,freeze the contents of the round-bottom flask for about 10minutes,open stopcock Afor 20seconds,and then close it.Remove the slurry bath,and repeat the heating steps as before,this time reaching a final temperature of between 45
(sufficient to liquefy most specimens)while stirring slowly but constantly with the magnetic bar.Cool the water in the bath by adding ice,and chill for about 2minutes.Replace the water bath with the slurry bath,freeze the contents of the round-bottom flask for about 10minutes,open stopcock Afor 20seconds,and then close it.Remove the slurry bath,and repeat the heating steps as before,this time reaching a final temperature of between 45 and 50
and 50 or a temperature necessary to melt the specimen completely.If there is any condensation in the tube connecting the round-bottom flask to the concentrator tube,slowly increase the voltage to the heating tape,and heat until condensation disappears.
or a temperature necessary to melt the specimen completely.If there is any condensation in the tube connecting the round-bottom flask to the concentrator tube,slowly increase the voltage to the heating tape,and heat until condensation disappears.
Stir with the magnetic stirrer throughout the following steps.Very slowly immerse the concentrator tube in a Dewar flask containing liquid nitrogen.[Caution—When there is liquid distillate in the concentrator tube,immerse the tube in the liquid nitrogen very slowly,or the tube will break.
]Water will begin to distill into the concentrator tube.As ice forms in the concentrator tube,raise the Dewar flask to keep the liquid nitrogen level only slightly below the level of ice in the tube.When water begins to freeze in the neck of the 10/30joint,or when liquid nitrogen reaches the 2.0-mLgraduation mark on the concentrator tube,remove the Dewar flask,and allow the ice to melt without heating.After the ice has melted,check the volume of water that has distilled,and repeat the sequence of chilling and thawing until not less than 0.9mLof water has been collected.Freeze the tube once again for about 2minutes,and release the vacuum first by opening stopcock B,followed by opening stopcock A.Remove the concentrator tube from the apparatus,close it with a greased stopper,and allow the ice to melt without heating.Mix the contents of the concentrator tube by swirling,note the volume of distillate,and dilute with water to 2.0mL,if necessary.
Chromatographic system
(see Chromatography á621ñ)—Use a gas chromatograph equipped with a flame-ionization detector.Under typical conditions,the instrument is equipped with a 2-mm ×1.8-m glass column that contains support S10.The column is maintained at a temperature of about 140 ,the injection port at 200
,the injection port at 200 ,and the detector at 250
,and the detector at 250 .Nitrogen or helium is the carrier gas,flowing at a rate of about 35mLper minute.Install an oxygen scrubber between the carrier gas line and the column.Condition the column for about 72hours at 230
.Nitrogen or helium is the carrier gas,flowing at a rate of about 35mLper minute.Install an oxygen scrubber between the carrier gas line and the column.Condition the column for about 72hours at 230 with 30to 40mLper minute carrier flow.[NOTE—Support S10is oxygen-sensitive.Each time a column is installed,flush with carrier gas for 30to 60minutes before heating.]
with 30to 40mLper minute carrier flow.[NOTE—Support S10is oxygen-sensitive.Each time a column is installed,flush with carrier gas for 30to 60minutes before heating.]
Procedure—
Separately inject equal volumes (about 2to 4µL)of the Standard solutionand the Test solution.The height of the peak in the chromatogram of the Test solutionis not greater than that in the chromatogram of the Standard not more than 10µg per g is found.
Assay—
Mobile phase—
Prepare a degassed solution containing a mixture of methanol and water (80:20),making adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Resolution solution—
Dissolve octoxynol 9and USP Nonoxynol 9RSin Mobile phaseto obtain a solution containing about 25mg of each per mL.
Standard preparation—
Dissolve an accurately weighed quantity of USP Nonoxynol 9RSin Mobile phaseto obtain a solution having a known concentration of about 25mg per mL.
Assay preparation—
Dissolve about 2.5g of Nonoxynol 9,accurately weighed,in Mobile phasein a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Chromatographic system—
The liquid chromatograph is equipped with a 280-nm detector and a 3.9-mm ×25-cm column that contains 10-µm packing L1.The flow rate is about 1mLper minute.Chromatograph the Resolution solution:the resolution,R,is not less than 2.0.Chromatograph replicate injections of the Standard preparation:the nonoxynol oligomers elute as a major peak,usually with shoulders and bumps.Include these in the peak response for Nonoxynol 9.The relative standard deviation is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for Nonoxynol 9,including any shoulders and bumps.Calculate the quantity,in mg,of Nonoxynol 9in the portion of specimen taken by the formula:
100C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Nonoxynol RSin the Standard preparation,and rUand rSare the peak responses of Nonoxynol 9obtained from the Assay and the Standard preparation,respectively.
*
Asuitable tube is available as Chromaflex concentrator tube,Kontes Glass Co.,Vineland,NJ(Catalog No.K42560-0000).
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 1390
Phone Number:1-301-816-8379