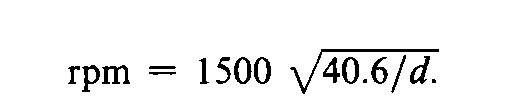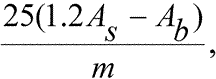á401ñFATS AND FIXED OILS
The following definitions and general procedures apply to fats,fixed oils,waxes,resins,balsams,and similar substances.
PREPARATION OF SPECIMEN
If a specimen of oil shows turbidity owing to separated stearin,warm the container in a water bath at 50 until the oil is clear,or if the oil does not become clear on warming,pass it through dry filter paper in a funnel contained in a hot-water jacket.Mix thoroughly,and weigh at one time as many portions as are needed for the various determinations,using preferably a bottle having a pipet dropper,or a weighing buret.Keep the specimen melted,if solid at room temperature,until the desired portions of specimen are withdrawn.
until the oil is clear,or if the oil does not become clear on warming,pass it through dry filter paper in a funnel contained in a hot-water jacket.Mix thoroughly,and weigh at one time as many portions as are needed for the various determinations,using preferably a bottle having a pipet dropper,or a weighing buret.Keep the specimen melted,if solid at room temperature,until the desired portions of specimen are withdrawn.
SPECIFIC GRAVITY
Determine the specific gravity of a fat or oil as directed under Specific Gravity á841ñ.
MELTING TEMPERATURE
Determine the melting temperature as directed for substances of Class II(see Melting Range or Temperature á741ñ).
ACID VALUE(FREE FATTY ACIDS)
The acidity of fats and fixed oils in this Pharmacopeia may be expressed as the number of mLof 0.1Nalkali required to neutralize the free acids in 10.0g of substance.Acidity is frequently expressed as the Acid Value,which is the number of mg of potassium hydroxide required to neutralize the free acids in 1.0g of the substance.
Procedure—
Unless otherwise directed,dissolve about 10.0g of the substance,accurately weighed,in 50mLof a mixture of equal volumes of alcohol and ether (which has been neutralized to phenolphthalein with 0.1Nsodium hydroxide)contained in a flask.If the test specimen does not dissolve in the cold solvent,connect the flask with a suitable condenser and warm slowly,with frequent shaking,until the specimen dissolves.Add 1mLof phenolphthalein TS,and titrate with 0.1Nsodium hydroxide VSuntil the solution remains faintly pink after shaking for 30seconds.Calculate either the Acid Value or the volume of 0.1Nalkali required to neutralize 10.0g of specimen (free fatty acids),whichever is appropriate.
If the volume of 0.1Nsodium hydroxide VSrequired for the titration is less than 2mL,a more dilute titrant may be used,or the sample size may be adjusted accordingly.The results may be expressed in terms of the volume of titrant used or in terms of the equivalent volume of 0.1Nsodium hydroxide.
If the oil has been saturated with carbon dioxide for the purpose of preservation,gently reflux the alcohol-ether solution for 10minutes before titration.The oil may be freed from carbon dioxide also by exposing it in a shallow dish in a vacuum desiccator for 24hours before weighing the test specimens.
ESTER VALUE
The Ester Value is the number of mg of potassium hydroxide required to saponify the esters in 1.0g of the substance.If the Saponification Valueand the Acid Valuehave been determined,the difference between these two represents the Ester Value.
Procedure—
Place 1.5g to 2g of the substance in a tared,250-mLflask,weigh accurately,add 20mLto 30mLof neutralized alcohol,and shake.Add 1mLof phenolphthalein TS,and titrate with 0.5Nalcoholic potassium hydroxide VSuntil the free acid is neutralized.Add 25.0mLof 0.5Nalcoholic potassium hydroxide VS,and proceed as directed under Saponification Value,beginning with “Heat the flask”and omitting the further addition of phenolphthalein TS.The difference between the volumes,in mL,of 0.5Nhydrochloric acid consumed in the actual test and in the blank test,multiplied by 28.05and divided by the weight in g of the specimen taken,is the Ester Value.
HYDROXYL VALUE
The Hydroxyl Value is the number of mg of potassium hydroxide equivalent to the hydroxyl content of 1.0g of the substance.
Pyridine–Acetic Anhydride Reagent—
Just before use,mix 3volumes of freshly opened or freshly distilled pyridine with 1volume of freshly opened or freshly distilled acetic anhydride.
Procedure—
Transfer a quantity of the substance,determined by reference to the accompanying tableand accurately weighed,to a glass-stoppered,250-mLconical flask,and add 5.0mLof Pyridine–Acetic Anhydride Reagent.Transfer 5.0mLof Pyridine–Acetic Anhydride Reagentto a second glass-stoppered,250-mLconical flask to provide the reagent blank.Fit both flasks with suitable glass-jointed reflux condensers,heat on a steam bath for 1hour,add 10mLof water through each condenser,and heat on the steam bath for 10minutes more.Cool,and to each add 25mLof butyl alcohol,previously neutralized to phenolphthalein TSwith 0.5Nalcoholic potassium hydroxide,by pouring 15mLthrough each condenser and,after removing the condensers,washing the sides of both flasks with the remaining 10-mLportions.To each flask add 1mLof phenolphthalein TS,and titrate with 0.5Nalcoholic potassium hydroxide VS,recording the volume,in mL,consumed by the residual acid in the test solution as Tand that consumed by the blank as B.In a 125-mLconical flask,mix about 10g of the substance,accurately weighed,with 10mLof freshly distilled pyridine,previously neutralized to phenolphthalein TS,add 1mLof phenolphthalein TS,and titrate with 0.5Nalcoholic potassium hydroxide VS,recording the volume,in mL,consumed by the free acid in the test specimen as A,or use the Acid Value to obtain A.Calculate the Hydroxyl Value taken by the formula:
(56.11N/W)[B+(WA/C)-T],
in which Wand Care the weights,in g,of the substances taken for the acetylation and for the free acid determination,respectively;Nis the exact normality of the alcoholic potassium hydroxide;and 56.11is the molecular weight of potassium hydroxide.
| Hydroxyl Value Range | Weight of Test Specimen,g |
| 0to 20 | 10 |
| 20to 50 | 5 |
| 50to 100 | 3 |
| 100to 150 | 2 |
| 150to 200 | 1.5 |
| 200to 250 | 1.25 |
| 250to 300 | 1.0 |
| 300to 350 | 0.75 |
IODINE VALUE
The Iodine Value represents the number of g of iodine absorbed,under the prescribed conditions,by 100g of the substance.Unless otherwise specified in the individual monograph,determine the Iodine Value by Method I.
Method I(Hanus Method)
Procedure—
Transfer an accurately weighed quantity of sample,as determined from the accompanying table,into a 250-mLiodine flask,dissolve it in 10mLof chloroform,add 25.0mLof iodobromide TS,insert the stopper in the vessel securely,and allow it to stand for 30minutes protected from light,with occasional shaking.Then add,in the order named,30mLof potassium iodide TSand 100mLof water,and titrate the liberated iodine with 0.1Nsodium thiosulfate VS,shaking thoroughly after each addition of thiosulfate.When the iodine color becomes quite pale,add 3mLof starch TS,and continue the titration with 0.1Nsodium thiosulfate VSuntil the blue color is discharged.Perform a blank test at the same time with the same quantities of the same reagents and in the same manner (see Residual Titrations á541ñ).Calculate the Iodine Value from the formula:
[126.9(VB-VS)N]/10W,
in which 126.9is the atomic weight of iodine;VBand VSare the volumes,in mL,of 0.1Nsodium thiosulfate VSconsumed by the blank test and the actual test,respectively;Nis the exact normality of the sodium thiosulfate VS;and Wis the weight,in g,of the substance taken for the test.
[NOTE—If more than half of the iodobromide TSis absorbed by the portion of the substance taken,repeat the determination,using a smaller portion of the substance under examination.]
Sample Weights
| Iodine value expected | Weight in g,±0.001 |
| <5 | 3.000 |
| 5–20 | 1.000 |
| 21–50 | 0.400 |
| 51–100 | 0.200 |
| 101–150 | 0.130 |
| 151–200 | 0.100 |
Method II
Potassium Iodide Solution—
Dissolve 10.0g of potassium iodide in water to make 100mL.Store in light-resistant containers.
Starch Indicator Solution—
Mix 1g of soluble starch with sufficient cold water to make a thin paste.Add,while stirring,to 100mLof boiling water.Mix,and cool.Use only the clear solution.
Procedure—
Melt the sample,if it is not already liquid.[NOTE—The temperature during melting should not exceed the melting point of the sample by more than 10 .]Pass through two pieces of filter paper to remove any solid impurities and the last traces of moisture.The filtration may be performed in an air oven at 100
.]Pass through two pieces of filter paper to remove any solid impurities and the last traces of moisture.The filtration may be performed in an air oven at 100 but should be completed within 5minutes ±30seconds.The sample must be absolutely dry.All glassware must be absolutely clean and completely dry.After filtration,allow the filtered sample to achieve a temperature of 68
but should be completed within 5minutes ±30seconds.The sample must be absolutely dry.All glassware must be absolutely clean and completely dry.After filtration,allow the filtered sample to achieve a temperature of 68 to 71±1
to 71±1 before weighing the sample.Once the sample has achieved a temperature of 68
before weighing the sample.Once the sample has achieved a temperature of 68 to 71±1
to 71±1 ,immediately weigh the sample into a 500-mLiodine flask,using the weights and weighing accuracy noted in the accompanying table.[NOTE—The weight of the substance must be such that there will be an excess of iodochloride TSof 50%to 60%of the amount added,that is,100%to 150%of the amount absorbed.]Add 15mLof a fresh mixture of cyclohexane and glacial acetic acid (1:1),and swirl to dissolve the sample.Add 25.0mLof iodochloride TS,insert the stopper securely in the flask,and swirl to mix.Allow it to stand at 25±5
,immediately weigh the sample into a 500-mLiodine flask,using the weights and weighing accuracy noted in the accompanying table.[NOTE—The weight of the substance must be such that there will be an excess of iodochloride TSof 50%to 60%of the amount added,that is,100%to 150%of the amount absorbed.]Add 15mLof a fresh mixture of cyclohexane and glacial acetic acid (1:1),and swirl to dissolve the sample.Add 25.0mLof iodochloride TS,insert the stopper securely in the flask,and swirl to mix.Allow it to stand at 25±5 ,protected from light,with occasional shaking,for 1.0or 2.0hours,depending on the Iodine Value (IV)of the sample:IVless than 150,1.0hour;IVequal to or greater than 150,2.0hours.Then,within 3minutes after the indicated reaction time,add,in the order named,20mLof Potassium Iodide Solutionand 150mLof recently boiled and cooled water,and mix.Within 30minutes,titrate the liberated iodine with 0.1Nsodium thiosulfate VS,while stirring by mechanical means after each addition of thiosulfate.When the yellow iodine color has almost disappeared,add 1to 2mLof Starch Indicator Solution,and continue the titration with 0.1Nsodium thiosulfate VSuntil the blue color is discharged.Perform a blank test at the same time with the same quantities of the same reagents and in the same manner (see Residual Titrations á541ñ).The difference between the volumes,in mL,of 0.1Nsodium thiosulfate consumed by the blank test and the actual test,multiplied by 1.269and divided by the weight,in g,of the sample taken,is the Iodine Value.
,protected from light,with occasional shaking,for 1.0or 2.0hours,depending on the Iodine Value (IV)of the sample:IVless than 150,1.0hour;IVequal to or greater than 150,2.0hours.Then,within 3minutes after the indicated reaction time,add,in the order named,20mLof Potassium Iodide Solutionand 150mLof recently boiled and cooled water,and mix.Within 30minutes,titrate the liberated iodine with 0.1Nsodium thiosulfate VS,while stirring by mechanical means after each addition of thiosulfate.When the yellow iodine color has almost disappeared,add 1to 2mLof Starch Indicator Solution,and continue the titration with 0.1Nsodium thiosulfate VSuntil the blue color is discharged.Perform a blank test at the same time with the same quantities of the same reagents and in the same manner (see Residual Titrations á541ñ).The difference between the volumes,in mL,of 0.1Nsodium thiosulfate consumed by the blank test and the actual test,multiplied by 1.269and divided by the weight,in g,of the sample taken,is the Iodine Value.
PEROXIDE VALUE
The Peroxide Value is the number that expresses,in milliequivalents of active oxygen,the quantity of peroxide contained in 1000g of the substance.[NOTE—This test must be performed promptly after sampling to avoid oxidation of the test specimen.]
Procedure—
Unless otherwise directed,place about 5g of the substance,accurately weighed,in a 250-mLconical flask fitted with a ground-glass stopper.Add 30mLof a mixture of glacial acetic acid and chloroform (3:2),shake to dissolve,and add 0.5mLof saturated potassium iodide solution.Shake for exactly 1minute,and add 30mLof water.Titrate with 0.01Nsodium thiosulfate VS,adding the titrant slowly with continuous shaking,until the yellow color is almost discharged.Add 5mLof starch TS,and continue the titration,shaking vigorously,until the blue color is discharged.Perform a blank determination under the same conditions.[NOTE—The volume of titrant used in the blank determination must not exceed 0.1mL.]The difference between the volumes,in mL,of 0.01Nsodium thiosulfate consumed in the actual test and in the blank test,multiplied by 10and divided by the weight,in g,of the specimen taken,is the Peroxide Value.
SAPONIFICATION VALUE
The Saponification Value is the number of mg of potassium hydroxide required to neutralize the free acids and saponify the esters contained in 1.0g of the substance.
Procedure—
Place 1.5g to 2g of the substance in a tared,250-mLflask,weigh accurately,and add to it 25.0mLof 0.5Nalcoholic potassium hydroxide.Heat the flask on a steam bath,under a suitable condenser to maintain reflux for 30minutes,frequently rotating the contents.Then add 1mLof phenolphthalein TS,and titrate the excess potassium hydroxide with 0.5Nhydrochloric acid VS.Perform a blank determination under the same conditions (see Residual Titrationsunder Titrimetry á541ñ).The titration also can be carried out potentiometrically.The difference between the volumes,in mL,of 0.5Nhydrochloric acid consumed in the actual test and in the blank test,multiplied by 56.1and the exact normality of the 0.5Nhydrochloric acid VS,and divided by the weight in g of specimen taken,is the Saponification Value.
If the oil has been saturated with carbon dioxide for the purpose of preservation,expose it in a shallow dish in a vacuum desiccator for 24hours before weighing the test specimens.
UNSAPONIFIABLE MATTER
The term “Unsaponifiable Matter”in oils or fats,refers to those substances that are not saponifiable by alkali hydroxides but are soluble in the ordinary fat solvents,and to products of saponification that are soluble in such solvents.
Procedure—
Transfer about 5.0g of the oil or fat,accurately weighed,to a 250-mLconical flask,add 50mLof an alcoholic potassium hydroxide solution prepared by dissolving 12g of potassium hydroxide in 10mLof water and diluting this solution with alcohol to 100mL,and heat the flask on a steam bath under a suitable condenser to maintain reflux for 1hour,swirling frequently.Cool to a temperature below 25 ,and transfer the contents of the flask to a separator having a polytetrafluoroethylene stopcock,rinsing the flask with two 50-mLportions of water that are added to the separator (do not use grease on stopcock).Extract with three 100-mLportions of ether,combining the ether extracts in another separator containing 40mLof water.Gently rotate or shake the separator for a few minutes.[NOTE—Violent agitation may result in the formation of a difficult-to-separate emulsion.]Allow the mixture to separate,and discard the lower aqueous phase.Wash the ether extract with two additional 40-mLportions of water,and discard the lower aqueous phase.Wash the ether extract successively with a 40-mLportion of potassium hydroxide solution (3in 100)and a 40-mLportion of water.Repeat this potassium hydroxide solution-water wash sequence three times.Wash the ether extract with 40-mLportions of water until the last washing is not reddened by the addition of 2drops of phenolphthalein TS.Transfer the ether extract to a tared flask,and rinse the separator with 10mLof ether,adding the rinsings to the flask.Evaporate the ether on a steam bath,and add 6mLof acetone to the residue.Remove the acetone in a current of air,and dry the residue at 105
,and transfer the contents of the flask to a separator having a polytetrafluoroethylene stopcock,rinsing the flask with two 50-mLportions of water that are added to the separator (do not use grease on stopcock).Extract with three 100-mLportions of ether,combining the ether extracts in another separator containing 40mLof water.Gently rotate or shake the separator for a few minutes.[NOTE—Violent agitation may result in the formation of a difficult-to-separate emulsion.]Allow the mixture to separate,and discard the lower aqueous phase.Wash the ether extract with two additional 40-mLportions of water,and discard the lower aqueous phase.Wash the ether extract successively with a 40-mLportion of potassium hydroxide solution (3in 100)and a 40-mLportion of water.Repeat this potassium hydroxide solution-water wash sequence three times.Wash the ether extract with 40-mLportions of water until the last washing is not reddened by the addition of 2drops of phenolphthalein TS.Transfer the ether extract to a tared flask,and rinse the separator with 10mLof ether,adding the rinsings to the flask.Evaporate the ether on a steam bath,and add 6mLof acetone to the residue.Remove the acetone in a current of air,and dry the residue at 105 until successive weighings differ by not more than 1mg.Calculate the percentage of unsaponifiable matter in the portion of oil or fat taken by the formula:
until successive weighings differ by not more than 1mg.Calculate the percentage of unsaponifiable matter in the portion of oil or fat taken by the formula:
100(WR/WS),
in which WRis the weight,in g,of the residue;and WSis the weight,in g,of the oil or fat taken for the test.
Dissolve the residue in 20mLof alcohol,previously neutralized to the phenolphthalein endpoint,add phenolphthalein TS,and titrate with 0.1Nalcoholic sodium hydroxide VSto the first appearance of a faint pink color that persists for not less than 30seconds.If the volume of 0.1Nalcoholic sodium hydroxide required is greater than 0.2mL,the separation of the layers was incomplete;the residue weighed cannot be considered as “unsaponifiable matter,”and the test must be repeated.
SOLIDIFICATION TEMPERATURE OF FATTY ACIDS
Preparation of the Fatty Acids—
Heat 75mLof glycerin–potassium hydroxide solution (made by dissolving 25g of potassium hydroxide in 100mLof glycerin)in an 800-mLbeaker to 150 ,and add 50mLof the clarified fat,melted if necessary.Heat the mixture for 15minutes with frequent stirring,but do not allow the temperature to rise above 150
,and add 50mLof the clarified fat,melted if necessary.Heat the mixture for 15minutes with frequent stirring,but do not allow the temperature to rise above 150 .Saponification is complete when the mixture is homogeneous,with no particles clinging to the beaker at the meniscus.Pour the contents of the beaker into 500mLof nearly boiling water in an 800-mLbeaker or casserole,add slowly 50mLof dilute sulfuric acid (made by adding water and sulfuric acid (3:1)),and heat the solution,with frequent stirring,until the fatty acids separate cleanly as a transparent layer.Wash the acids with boiling water until free from sulfuric acid,collect them in a small beaker,place on a steam bath until the water has settled and the fatty acids are clear,filter into a dry beaker while hot,and dry at 105
.Saponification is complete when the mixture is homogeneous,with no particles clinging to the beaker at the meniscus.Pour the contents of the beaker into 500mLof nearly boiling water in an 800-mLbeaker or casserole,add slowly 50mLof dilute sulfuric acid (made by adding water and sulfuric acid (3:1)),and heat the solution,with frequent stirring,until the fatty acids separate cleanly as a transparent layer.Wash the acids with boiling water until free from sulfuric acid,collect them in a small beaker,place on a steam bath until the water has settled and the fatty acids are clear,filter into a dry beaker while hot,and dry at 105 for 20minutes.Place the warm fatty acids in a suitable container,and cool in an ice bath until they congeal.
for 20minutes.Place the warm fatty acids in a suitable container,and cool in an ice bath until they congeal.
Test for Complete Saponification—
Place 3mLof the dry acids in a test tube,and add 15mLof alcohol.Heat the solution to boiling,and add an equal volume of 6Nammonium hydroxide.Aclear solution results.
Procedure—
Using an apparatus similar to the “Congealing Temperature Apparatus”specified therein,proceed as directed for Procedureunder Congealing Temperature á651ñ,reading “solidification temperature”for “congealing point”(the terms are synonymous).The average of not less than four consecutive readings of the highest point to which the temperature rises is the solidification temperature of the fatty acids.
FATTY ACID COMPOSITION
Standard Solution—
Prepare an ester mixture of known composition containing the esters required in the individual monograph.This Standard Solutionmay contain other components.[NOTE—Ester mixtures are available commercially from Nu-Chek-Prep,Inc.,P.O.Box 295,Elysian,MN56028.Typical Nu-Chek-Prep ester mixtures useful in this test include Nu-Chek 17Aand Nu-Chek 19A.]Nu-Chek mixture 17Ahas the following composition:
| Percentage | Fatty Acid Ester | Carbon-chain Length | No.of Double Bonds |
| 1.0 | methyl myristate | 14 | 0 |
| 4.0 | methyl palmitate | 16 | 0 |
| 3.0 | methyl stearate | 18 | 0 |
| 3.0 | methyl arachidate | 20 | 0 |
| 3.0 | methyl behenate | 22 | 0 |
| 3.0 | methyl lignocerate | 24 | 0 |
| 45.0 | methyl oleate | 18 | 1 |
| 15.0 | methyl linoleate | 18 | 2 |
| 3.0 | methyl linolenate | 18 | 3 |
| 20.0 | methyl erucate | 22 | 1 |
Nu-Chek mixture 19Ahas the following composition:
| Percentage | Fatty Acid Ester | Carbon-chain Length | No.of Double Bonds |
| 7.0 | methy caprylate | 8 | 0 |
| 5.0 | methyl caprate | 10 | 0 |
| 48.0 | methyl laurate | 12 | 0 |
| 15.0 | methyl myristate | 14 | 0 |
| 7.0 | methyl palmitate | 16 | 0 |
| 3.0 | methyl stearate | 18 | 0 |
| 12.0 | methyl oleate | 18 | 1 |
| 3.0 | methyl linoleate | 18 | 2 |
Test Solution—
[NOTE—If fatty acids containing more than 2double bonds are present in the test specimen,remove air from the flask by purging it with nitrogen for a few minutes.]Transfer about 100mg of the test specimen to a 50-mLconical flask fitted with a suitable water-cooled reflux condenser and a magnetic stir bar.Add 4mLof 0.5Nmethanolic sodium hydroxide solution,and reflux until fat globules disappear (usually 5to 10minutes).Add 5mLof a solution prepared by dissolving 14g of boron trifluoride in methanol to make 100mL,swirl to mix,and reflux for 2minutes.Add 4mLof chromatographic n-heptane through the condenser,and reflux for 1minute.Cool,remove the condenser,add about 15mLof saturated sodium chloride solution,shake,and allow the layers to separate.Pass the n-heptane layer through 0.1g of anhydrous sodium sulfate (previously washed with chromatographic n-heptane)into a suitable flask.Transfer 1.0mLof this solution to a 10-mLvolumetric flask,dilute with chromatographic n-heptane to volume,and mix.
System Suitability Solution—
Transfer about 20mg each of stearic acid,palmitic acid and oleic acid to a 25-mLconical flask fitted with a suitable water-cooled reflux condenser and a magnetic stir bar,and proceed as directed for Test Solution,beginning with “Add 5.0mLof a solution prepared by dissolving.”
Chromatographic System (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector,maintained at a temperature of about 260 ,a splitless injection system,and a 0.53-mm ×30-m fused-silica capillary column bonded with a 1.0-µm layer of phase G16.The chromatograph is programmed to maintain the column temperature at 70
,a splitless injection system,and a 0.53-mm ×30-m fused-silica capillary column bonded with a 1.0-µm layer of phase G16.The chromatograph is programmed to maintain the column temperature at 70 for about 2minutes after injection,then to increase the temperature at the rate of 5
for about 2minutes after injection,then to increase the temperature at the rate of 5 per minute to 240
per minute to 240 ,and finally to maintain this temperature for 5minutes.The injection port temperature is maintained at about 220
,and finally to maintain this temperature for 5minutes.The injection port temperature is maintained at about 220 .The carrier gas is helium with a linear velocity of about 50cm per second.
.The carrier gas is helium with a linear velocity of about 50cm per second.
Chromatograph the System Suitability Solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.87for methyl palmitate,0.99for methyl stearate,and 1.0for methyl oleate;the resolution,R,between methyl stearate and methyl oleate is not less than 1.5;and the relative standard deviation of the peak area responses for the palmitate and stearate peaks for replicate injections is not more than 6.0%.The relative standard deviation of the peak area response ratio of the palmitate to stearate peaks from these replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 1µL)of the Standard Solutionand the Test Solutioninto the chromatograph,record the chromatograms,identify the fatty acid ester peaks in the chromatogram of the Test Solutionby comparing the retention times of these peaks with those obtained in the chromatogram of the Standard Solution,and measure the peak areas for all of the fatty acid ester peaks in the chromatogram obtained from the Test Solution.Calculate the percentage of each fatty acid component in the test specimen by the formula:
100(A/B),
in which Ais the area of the peak response obtained for each individual fatty acid ester component;and Bis the sum of the peak areas of all of the peaks,excluding the solvent peak,in the chromatogram obtained from the Test Solution.
WATER AND SEDIMENT IN FIXED OILS
Apparatus—
The preferred centrifuge has a diameter of swing (d=distance from tip to tip of whirling tubes)of 38to 43cm and is operated at a speed of about 1500rpm.If a centrifuge of different dimensions is used,calculate the desired rate of revolution by the formula:
The centrifuge tubes are pear-shaped,and are shaped to accept closures.The total capacity of each tube is about 125mL.The graduations are clear and distinct,reading upward from the bottom of the tube according to the scale shown in the accompanying table.
| Volume (mL) | Scale Division (mL) |
| 0to 3 | 0.1 |
| 3to 5 | 0.5 |
| 5to 10 | 1.0 |
| 10to 25 | 5.0 |
| 25to 50 | 25.0 |
| 50to 100 | 50.0 |
Procedure—
Place 50.0mLof benzene in each of two centrifuge tubes,and to each tube add 50.0mLof the oil,warmed if necessary to re-incorporate separated stearin,and mixed thoroughly at 25 .Insert the stopper tightly into the tubes,and shake them vigorously until the contents are mixed thoroughly,then immerse the tubes in a water bath at 50
.Insert the stopper tightly into the tubes,and shake them vigorously until the contents are mixed thoroughly,then immerse the tubes in a water bath at 50 for 10minutes.Centrifuge for 10minutes.Read the combined volume of water and sediment at the bottom of each tube.Centrifuge repeatedly for 10-minute periods until the combined volume of water and sediment remains constant for 3consecutive readings.The sum of the volumes of combined water and sediment in the two tubes represents the percentage,by volume,of water and sediment in the oil.
for 10minutes.Centrifuge for 10minutes.Read the combined volume of water and sediment at the bottom of each tube.Centrifuge repeatedly for 10-minute periods until the combined volume of water and sediment remains constant for 3consecutive readings.The sum of the volumes of combined water and sediment in the two tubes represents the percentage,by volume,of water and sediment in the oil.
ANISIDINE VALUE
The anisidine value is defined as 100times the optical density measured in a 1-cm cell of a solution containing 1g of the substance to be examined in 100mLof a mixture of solvents and reagents according to the method described below.[NOTE—Carry out the operations as rapidly as possible,avoiding exposure to actinic light.]
Test Solution A—
Dissolve 0.500g of the substance to be examined in isooctane,and dilute with the same solvent to 25.0mL.
Test Solution B—
To 5.0mLof Test Solution Aadd 1.0mLof a 2.5g per Lsolution of p-anisidine in glacial acetic acid,shake,and store protected from light.
Standard Solution—
To 5.0mLof isooctane add 1.0mLof a 2.5g per Lsolution of p-anisidine in glacial acetic acid,shake,and store protected from light.
Procedure—
Measure the absorbance of Test Solution Aat 350nm using isooctane as the blank.Measure the absorbance of Test Solution Bat 350nm exactly 10minutes after its preparation,using the Standard Solutionas the compensation liquid.Calculate the Anisidine Value from the expression:
in which Asis the absorbance of Test Solution Bat 350nm;Abis the absorbance of Test Solution Aat 350nm;and mis the weight,in g,of the substance to be examined in Test Solution A.
TOTAL OXIDATION VALUE(TOTOX)
Total Oxidation Value is defined by the formula:
2PV+AV,
in which PVis the Peroxide Value,and AVis the Anisidine Value.