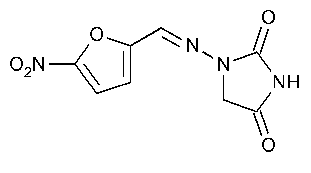
Nitrofurantoin
2,4-Imidazolidinedione,1-[[(5-nitro-2-furanyl)methylene]amino]-.
1-[(5-Nitrofurfurylidene)amino]hydantoin [67-20-9].
Monohydrate 256.18 [17140-81-7].
»Nitrofurantoin is anhydrous or contains one molecule of water of hydration.It contains not less than 98.0percent and not more than 102.0percent of C8H6N4O5,calculated on the anhydrous basis.
NOTE—Nitrofurantoin and solutions of it are discolored by alkali and by exposure to light,and are decomposed upon contact with metals other than stainless steel and aluminum.
Packaging and storage—
Preserve in tight,light-resistant containers.
Labeling—
Label it to indicate whether it is anhydrous or hydrous.Nitrofurantoin in the form of macrocrystals is so labeled.The labeling states the specific surface area and which method,specified under Specific Surface Area á846ñ,is used.
USP Reference standards á11ñ—
USP Nitrofurantoin RS.USP Nitrofurazone RS.USP Nitrofurfural Diacetate RS.
Identification—
A:
Infrared Absorption á197Mñ:previously dried at 140 for 30minutes.
for 30minutes.
B:
The retention time of the major peak in the chromatogram of the Assay preparationcorresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
Water,Method IIIá921ñ—
Dry it at 140 for 30minutes:the anhydrous form loses not more than 1.0%,and the hydrous form between 6.5%and 7.5%,of its weight.
for 30minutes:the anhydrous form loses not more than 1.0%,and the hydrous form between 6.5%and 7.5%,of its weight.
Specific surface area á846ñ(where it is labeled as being in the form of macrocrystals)—
Outgas a portion of the powder to be placed under test at 150 for 10minutes at ambient pressure with nitrogen:the limits are between 0.045m2per g and 0.20m2per g.
for 10minutes at ambient pressure with nitrogen:the limits are between 0.045m2per g and 0.20m2per g.
Limit of nitrofurfural diacetate—
In a 10-mLvolumetric flask dissolve 100mg of Nitrofurantoin in 1mLof dimethylformamide,add acetone to volume,and mix.Apply 10µLof this solution and 10µLof a Standard solution of USP Nitrofurfural Diacetate RSin a 1in 10mixture of dimethylformamide in acetone containing 100µg per mLto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Allow the spots to dry,and develop the chromatogram in a solvent system consisting of a mixture of chloroform and methanol (9:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,allow it to air-dry for 5minutes,and heat the plate at 105 for 5minutes.Remove the plate from the oven and,while it is still warm,locate the spots by spraying the plate with a solution prepared by dissolving 0.75g of phenylhydrazine hydrochloride in 50mLof water,decolorizing with activated charcoal,adding 25mLof hydrochloric acid,and mixing with water to produce 200mL.Any spot produced by the test specimen,at an RFvalue of about 0.7,is not greater in size or intensity than that produced by the Standard solution at the same RFvalue:not more than 1.0%of nitrofurfural diacetate is found.
for 5minutes.Remove the plate from the oven and,while it is still warm,locate the spots by spraying the plate with a solution prepared by dissolving 0.75g of phenylhydrazine hydrochloride in 50mLof water,decolorizing with activated charcoal,adding 25mLof hydrochloric acid,and mixing with water to produce 200mL.Any spot produced by the test specimen,at an RFvalue of about 0.7,is not greater in size or intensity than that produced by the Standard solution at the same RFvalue:not more than 1.0%of nitrofurfural diacetate is found.
Limit of nitrofurazone—
pH7.0Phosphate buffer—
Prepare as directed in the Assay.
Mobile phase—
Prepare a filtered and degassed mixture of pH7.0Phosphate bufferand tetrahydrofuran (9:1).
Standard preparation—
Prepare a solution of USP Nitrofurazone RSin dimethylformamide containing 5.0µg per mL.Pipet 2mLof this solution into a glass-stoppered flask,add 20.0mLof water,and mix.
Test preparation—
Dissolve 100mg of Nitrofurantoin in 2.0mLof dimethylformamide in a glass-stoppered,25-mLflask.Add 20.0mLof water,mix,and allow to stand for about 15minutes to allow precipitate to form.Pass a portion of the solution through a nylon filter having a 0.45-µm porosity,and use the clear filtrate.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 375-nm detector and a 3.9-mm ×30-cm column containing packing L1.The flow rate is about 1.6mLper minute.Chromatograph the Standard preparation,adjusting the operating parameters so that the nitrofurazone peak has a retention time of about 10.5minutes and its height is about 0.1full-scale.The relative standard deviation determined from the peak height in replicate injections is not more than 2.0%.Prepare a solution containing about 5.0µg each of nitrofurazone and nitrofurantoin per mLin dimethylformamide.Dilute this solution 1:10with Mobile phase,and inject 60µLto 100µL:the resolution,R,of the two peaks is not less than 4.0.
Procedure—
Separately inject equal volumes (60µLto 100µL)of the Standard preparationand the Test preparationinto the chromatograph,and record the chromatograms.The height of any peak appearing in the chromatogram of the Test preparationat a retention time corresponding to that of the main peak from the Standard preparationis not greater than the height of the main peak from the Standard preparation:not more than 0.01%of nitrofurazone is found.
Assay—
pH7.0Phosphate buffer—
Dissolve 6.8g of monobasic potassium phosphate in about 500mLof water.Add a volume of 1.0Nsodium hydroxide (about 30mL)sufficient to adjust to a pHof 7.0,dilute with water to 1liter,and mix.
Mobile phase—
Prepare a filtered and degassed mixture of pH7.0Phosphate bufferand acetonitrile (88:12).
Internal standard solution—
Prepare a solution containing about 1mg of acetanilide per mLin water,and mix.
Standard preparation—
Dissolve about 50mg of USP Nitrofurantoin RS,accurately weighed,in 40.0mLof dimethylformamide in a glass-stoppered flask,add 50.0mLof Internal standard solution,and mix.
Assay preparation—
Using about 50mg of Nitrofurantoin,accurately weighed,proceed as directed for Standard preparation.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm ×30-cm column that contains packing L1.Chromatograph the Standard preparation,adjusting the operating parameters so that the retention time of the nitrofurantoin peak is about 8minutes and the peak heights are about half full-scale.The resolution,R,between acetanilide and nitrofurantoin is not less than 3.0,and the relative standard deviation determined from the ratio of the peak responses in replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (5µLto 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C8H6N4O5in the portion of Nitrofurantoin taken by the formula:
W(RU/RS),
in which Wis the weight,in mg,of USP Nitrofurantoin RSin the Standard preparation;and RUand RSare the peak response ratios obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1380
Pharmacopeial Forum:Volume No.28(3)Page 717
Phone Number:1-301-816-8394