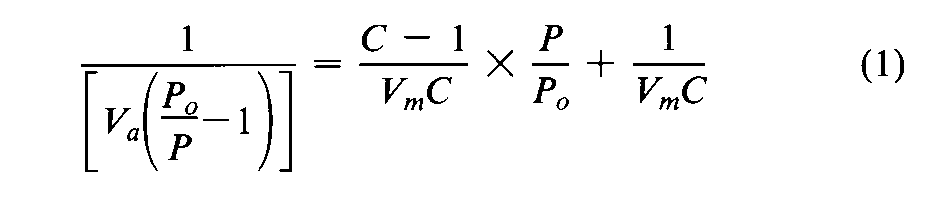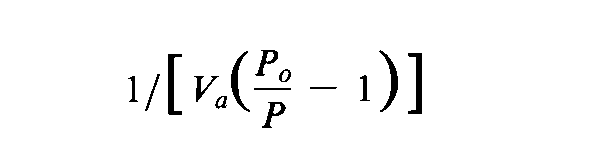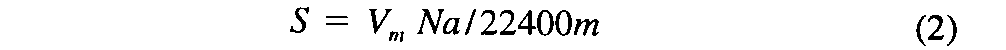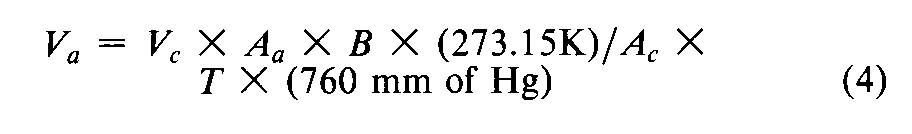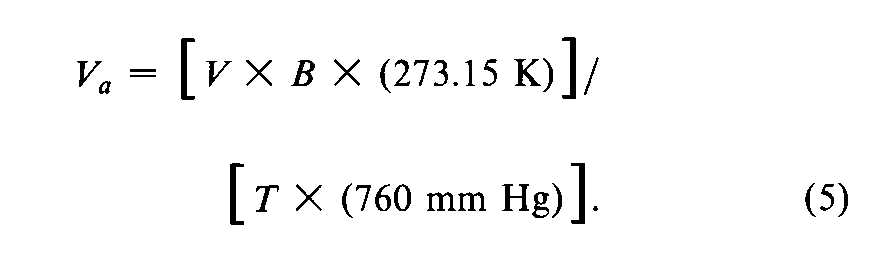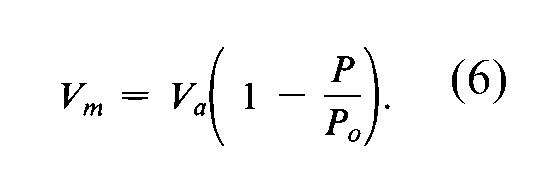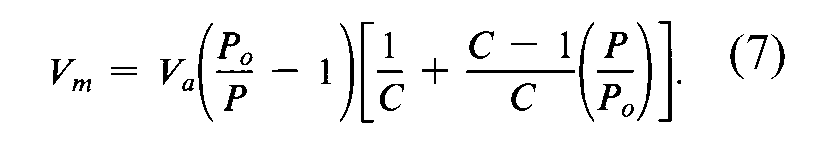á846ñSPECIFIC SURFACE AREA
Introduction— The specific surface area of a powder is determined by physical adsorption of a gas on the surface of the solid and by measuring the amount of adsorbate gas corresponding to a single layer (monolayer)on the surface.Physical adsorption results from relatively weak forces (van der Waals forces)between the adsorbate gas molecules and the adsorbent surface of the test powder.The amount of gas adsorbed can be measured by a gravimetric,volumetric,or continuous flow procedure.Since the amount of gas adsorbed under a given pressure tends to increase on decreasing the temperature,adsorption measurements are usually made at a low temperature.Measurement is performed at 77K,the boiling point of liquid nitrogen.The data are treated according to the Brunauer,Emmett,and Teller (BET)adsorption isotherm equation: where P,in mm of mercury,is the partial vapor pressure of adsorbate gas in equilibrium with the surface at 77K,Po,in mm of mercury,is the saturated pressure of the adsorbate gas,Va,in mL,is the volume of gas adsorbed at standard temperature and pressure (STP,273.15Kand 760mm of mercury),Vm,in mL,is the volume of gas adsorbed at STPto produce an apparent monolayer on the sample surface,and Cis a dimensionless constant that is related to the enthalpy of adsorption of the adsorbate gas on the powder sample.Avalue of Vais measured at each of not less than three values of P/Po.Then the BETvalue is plotted against P/Poaccording to equation (1).This plot should yield a straight line.The data are considered acceptable if the correlation coefficient,r,of the linear regression is not less than 0.9975;that is,r 2is not less than 0.995.From the resulting linear plot,the slope,which is equal to (C-1)/VmC,and the intercept,which is equal to 1/VmC,are evaluated by linear regression analysis.From these values,Vmis calculated as 1/(slope+intercept),while Cis calculated as (slope/intercept)+1.From the value of Vmso determined,the specific surface area,S,in m2g-1,is calculated by the equation: in which Nis the Avogadro constant (6.023×1023mole-1),ais the effective cross-sectional area of one adsorbate molecule (0.162×10-18m2for nitrogen or 0.195×10-18m2for krypton),mis the mass,in g,of test powder,and 22400is the volume,in mL,occupied by 1mole of the adsorbate gas at STP,allowing for minor departures from ideality.
Some calculations and procedures,described below,require the absolute value of Po,defined above,and of the barometric pressure,B,in mm of mercury.For liquid nitrogen open to the atmosphere in a Dewar vessel,Poand Bare taken to be equal to the measured atmospheric pressure (i.e.,barometric pressure)plus 10mm of mercury.Some instruments automatically perform the above measurements and computations.The upward correction of Poand Ballows for the elevation of the boiling point of nitrogen caused by impurities,such as oxygen,dissolved in the liquid nitrogen in the open Dewar vessel.This chapter describes the dynamic flow gas adsorption technique (Method I)and the volumetric gas adsorption technique (Method II).
Outgassing
Before the specific surface area of the sample can be determined,it is necessary to remove gases and vapors that may have become physically adsorbed onto the surface after manufacture and during treatment,handling,and storage.If outgassing is not achieved,the specific surface area may be reduced or may be variable because an intermediate area of the surface is covered with molecules of the previously adsorbed gases or vapors.
Conditions—
The outgassing conditions must be demonstrated to yield reproducible BETplots,as discussed under Introduction,a constant weight of test powder,and no detectable physical or chemical changes in the test powder.The outgassing conditions are critical for obtaining the required precision and accuracy of specific surface area measurements on pharmaceuticals because of the sensitivity of the surfaces of the materials.The outgassing conditions defined by the temperature,pressure,and time should be so chosen that the original surface of the solid is reproduced as closely as possible.Outgassing of many substances is often achieved by applying a vacuum or by purging the sample in a flowing stream of a nonreactive gas.In either case,elevated temperatures are sometimes applied to increase the rate at which the contaminants leave the surface.Outgassing by heating the test powder may change the nature of the surface and should be avoided unless specifically indicated in the individual monograph.The outgassing conditions stated in the monograph for each material have been derived where possible from studies of several materials from various manufacturers.If heating is employed,the recommended temperature and time of outgassing are as low as possible so as to achieve reproducibly high measures of specific surface area within an acceptable time span.For outgassing sensitive specimens the desorption-adsorption cycling method may be employed.
Procedure—
To outgas the test specimen by the desorption-adsorption cycling method,proceed as follows.Allow the test powder to equilibrate with the adsorbate gas,nitrogen at P/Po=0.30or krypton at P/Po=1.038×10-3,for at least one minute.Raise the Dewar vessel containing liquid nitrogen at 77Kup to a defined point on the sample cell.This level should be high enough above the level of the sample to ensure that the temperature drops to 77Kyet below any connections,such as O-rings in the cell holder,which could leak if cooled.At least one minute after vigorous boiling of liquid nitrogen has subsided,record or zero the gas detector-integrator signal according to Method I,or record the pressure signal according to Method II(adsorption signal).Lower the Dewar vessel from the sample cell,and surround the sample cell with a beaker of water at room temperature.Record the gas detector-integrator signal according to Method I,or record the pressure signal according to the Method II(desorption signal)when the signal has become constant.Repeat the cooling-heating cycle until three successive desorption signals differ by not more than 3%.If possible,reweigh the sealed sample cell containing the test powder and subtract the mass of the empty sample cell to obtain the actual mass,m,of the test powder.The sealed sample cell containing the test powder may be weighed after the measurement of the specific surface area,if necessary.
Standard Materials
The method employed for the determination of the specific surface area of the samples should be tested using reference standards of known surface area,such as alpha alumina,purchased together with a certificate of analysis from a scientifically accredited source.*The chosen reference standard should have a specific surface area as similar as possible to that of the powder sample and should be treated with the utmost care.If the measured specific surface area of the reference material does not fall within the range specified on the certificate of analysis from an accredited source,the same adsorbate gas being used,the elements of the method and the gas adsorption apparatus should be carefully examined to identify the cause of the discrepancy,which should then be eliminated.
METHOD I—THE DYNAMIC FLOW METHOD
Principles of the Method—
In the dynamic flow method,the recommended adsorbate gas,is nitrogen or krypton,while helium is employed as a diluent gas,which is not adsorbed under the recommended conditions.For powder samples of surface area less than about 0.3m2(or of specific surface area less than about 0.5m2g-1)krypton is the preferred adsorbate gas,while for samples of greater surface area,nitrogen is preferred.Aminimum of three mixtures of the appropriate adsorbate gas with helium are required within the P/Porange 0.05to 0.30,such as 0.100,0.200,and 0.300mole fraction of nitrogen or 3.46×10-4,6.92×10-4,and 1.038×10-3mole fraction of krypton,where P/Pois the relative pressure of the adsorbate gas,Pbeing the partial vapor pressure and Pobeing the saturated vapor pressure of the adsorbate gas.These mixtures should be certified to within 1%(absolute)or may be obtained by using a suitable apparatus to mix the appropriate adsorbate gas with helium in proportions accurate to 1%.Nitrogen or krypton,at least 99.9mole percent,is also required for calibrating the gas detector-integrator employed for measuring the volume or mass of the gas adsorbed,or desorbed,by the test powder.The gas detector-integrator should provide a signal that is approximately proportional to the volume of the gas passing through it under defined conditions of temperature and pressure.For this purpose,a thermal conductivity detector with an electronic integrator is one among various suitable types.Aminimum of three data points within the recommended range of 0.05to 0.30for P/Poshould be determined.
Procedure—
Accurately weigh a quantity of the test powder,such that the total surface area is at least 1m2,if possible,in a tared gas adsorption flow cell.[NOTE—Transfer the test powder carefully into the flow cell so that a clear path is provided for the flow of the gas.An approximate value of the mass,m,of the test powder to be taken may be calculated from the mean particle diameter,d,and the true or crystal density,r,assuming spherical particles by the equation:
in which Ais the surface area desired.Because of the assumptions on which it is based,this equation must not be employed to calculate the actual surface area.]Outgas the powder sample.
Pass the gas mixture containing the largest mole fraction of the adsorbate gas,nitrogen or krypton,within the recommended range over the test powder for at least 30seconds.Raise the Dewar vessel containing liquid nitrogen at 77Kup to a defined point on the flow cell.As mentioned above,this level should be high enough above the level of the sample to ensure that the temperature drops to 77Kyet below any connections,such as O-rings in the cell holder,which could leak if cooled.Record or zero the gas detector-integrator signal (adsorption signal)at least one minute after vigorous boiling of liquid nitrogen has subsided.Lower the Dewar vessel from the flow cell,and surround the flow cell with a beaker of water at room temperature.Record the gas detector-integrator signal (desorption signal)when the signal becomes constant.Repeat the cooling-heating cycle until three successive desorption signals differ by not more than 3%.The data for the first point may be derived from the desorption-adsorption cycling method,provided that this method is employed for outgassing the powder sample.
Record the last three desorption values at the largest mole fraction of the adsorbate gas,and calculate the arithmetic mean.Calibrate the gas detector-integrator by injecting,from a gas-tight syringe,a known volume of adsorbate gas,measured to ±2%,so as to give a signal within 15%of the last three desorption signals.Calculate the corresponding volume of adsorbate gas,Va(in mm of mercury),at standard temperature and pressure (STP,273.15Kand 760mm of mercury),from the calibrated detector-integrator signal by the equation:
in which Vcis the volume,in mL,of gas injected for calibration,Aais the detector-integrator response for the gas desorbed from the test powder,Acis the detector-integrator signal response for the calibration volume,Bis the measured barometric pressure,in mm of mercury without the correction (described above under Introduction),and T,in K,is the temperature of volume measurement (room temperature).If possible,weigh the sealed flow cell containing the test powder,and subtract the mass of the empty flow cell from this mass to obtain the actual mass,m,of the powder taken.
Prepare at least two other mixtures of the appropriate adsorbate gas with helium,as described above.For each of these gas mixtures,repeat the above cooling-heating cycle,beginning with “Pass the gas mixture.”Repeat the measurements for each of the gas mixtures until three successive desorption signals differ by not more than 3%.Immediately after recording each gas mixture signal,calibrate the gas detector-integrator,and calculate Vaas directed in the previous paragraph.
If the BETplot is not linear,when using nitrogen as the adsorbate gas,or if the measured amounts of nitrogen adsorbed are not repeatable to within ±3%,lack of strict proportionality between the measured values and the actual amounts of nitrogen adsorbed may be suspected,perhaps because of the thermal diffusion effect of nitrogen.The magnitude of this effect increases,and the accuracy and precision of the measurements decrease,with decreasing specific surface area of the powder specimen.Under these circumstances,and especially if the surface area of the powder sample is less than 0.3m2or if the specific surface area of the powder sample is less than 0.5m2g-1,as mentioned above,the thermal diffusion effect may be considerably reduced or eliminated by using an adsorbate gas that has a lower vapor pressure,such as krypton,instead of nitrogen.Since for krypton the saturated vapor pressure at 77Kis only 2.63mm of mercury,the required mole fractions of krypton in the three mixtures of krypton with helium are less than those of nitrogen in its mixtures with helium by a factor of 2.63/760=0.00346.Since the cross-sectional area of the krypton molecule is not well defined,it is recommended that the standard value,a=0.195nm2,be used in equation (2)when calculating the specific surface area,and that the name of the adsorbate gas employed be stated,if it is not nitrogen,when reporting the value of the specific surface area.
METHOD II—THE VOLUMETRIC METHOD
Principles of the Method—
In the volumetric method,the recommended adsorbate gas is nitrogen which is admitted into the evacuated space above the previously outgassed powder sample to give a defined equilibrium pressure,P,of the gas.The use of a diluent gas,such as helium,is therefore unnecessary,although helium may be employed for other purposes,such as to measure the void volume.Since only pure adsorbate gas,instead of a gas mixture,is employed,interfering effects of thermal diffusion are avoided in this method.Consequently,the use of an adsorbate gas possessing a low vapor pressure at 77K,such as krypton,is unnecessary.Some instruments employ a balance tube to offset the effects of free space,thermal gradients,and non-ideal gas behavior.Other instruments claim other advantages.The use of equipment from any particular manufacturer is not specifically endorsed.
The volume of nitrogen admitted into the sample tube to give the equilibrium pressure,P,is equal to the sum of the volume of gas actually adsorbed,V,plus the volume of gas in the free space around and above the sample,Vf,which must be either corrected for by suitable adjustment of the instrument or balanced out.Vis then converted by the following equation to the volume,Va,occupied by the same amount of gas at standard temperature and pressure (STP,273.15Kand 760mm of mercury):
Several instruments automatically perform the above measurements and computations.The instrument manual should always be consulted for guidance and for a complete description of the procedures for data acquisition and computation.Avalue of Vais measured at each of not less than three values of P/Po,and the data are plotted so as to provide a value of the specific surface area as described above under Introduction.
Procedure—
Accurately weigh a quantity of the test powder,such that the total surface area is at least 1m2,if possible,in a tared gas adsorption tube.[To calculate an approximate value of the mass of the test powder to be taken,use equation (3).]Admit a small amount of dry nitrogen into the sample tube to prevent contamination of the clean surface,remove the sample tube,insert the stopper,and weigh it.Calculate the weight of the sample.Attach the sample tube to the dynamic volumetric apparatus.Cautiously evacuate the sample down to a pressure of 0.02mm of mercury or less.
If the principle of operation of the instrument requires the determination of the void volume in the sample tube,for example,by the admission of a nonadsorbed gas,such as helium,this procedure is carried out at this point,followed by evacuation of the sample down to 0.02mm of mercury or less.The adsorption of nitrogen gas is then measured as described below.
Raise a Dewar vessel containing liquid nitrogen at 77Kup to a defined point on the sample cell as directed under Method I.Admit a sufficient volume of nitrogen gas to give a relative pressure,P/Po,equal to 0.10±0.02.Measure the volume adsorbed,Va.Repeat the measurement of Vaat P/Povalues of 0.20±0.02and 0.30±0.02.Aminimum of three data points is required.Additional measurements may be carried out,especially on those rare occasions when nonlinearity is obtained at a P/Povalue close to 0.3.Since nonlinearity is often obtained at P/Poat or below 0.05,values in this region are not recommended.The test for linearity,the treatment of the data,and the calculation of the specific surface area of the sample are described above under Introduction.
Single-Point Measurement
Normally,at least three measurements of Va,each at a different value of P/Po,are required for the determination of specific surface area by Method Ior Method II.However,under certain circumstances it may be acceptable to determine the specific surface area of a powder from a single value of Vameasured at a single value of P/Po,such as 0.300(corresponding to 0.300mole of nitrogen or 0.001038mole fraction of krypton),using the following equation for calculating Vm:
The single-point method may be employed directly for a series of powder samples of a given material for which the material constant,C,is much greater than unity.This circumstance may be verified by comparing values of specific surface area determined by the single-point method with that determined by the multiple-point method for the series of powder samples.Close similarity between the single-point values and multiple-point values suggests that 1/Capproaches zero.
The single-point method may be employed indirectly for a series of very similar powder samples of a given material for which the material constant,C,is not infinite but may be assumed to be invariant.Under this circumstance,the error associated with the single-point method can be reduced or eliminated by using the multiple-point method to evaluate Cfor one of the samples of the series from the BETplot,from which Cis calculated as (1+slope/intercept).Then Vmis calculated from the single value of Vameasured at a single value of P/Poby the equation:
The specific surface area is calculated from Vmby equation (2)stated above.
*
Asuitable scientifically accredited source is the National Institute of Standards and Technology,Gaithersburg,Maryland.