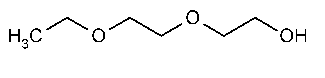
Diethylene Glycol Monoethyl Ether
»Diethylene Glycol Monoethyl Ether contains not less than 99.0percent and not more than 101.0percent of C6H14O3.It is produced by condensation of ethylene oxide and alcohol,followed by distillation.
Packaging and storage—
Preserve in tight containers under an atmosphere of an inert gas,at a temperature not exceeding 35 .
.
Labeling—
The label indicates that it is stored under an atmosphere of an inert gas.
Identification—
A:
Infrared Absorption á197Fñ,potassium bromide plates being used.
B:
The retention time of the major peak in the chromatogram of the Assay preparationcorresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
Refractive index á831ñ:
between 1.426and 1.428at 20 .
.
Water,Method Iá921ñ:
not more than 0.1%,determined on a 10-g specimen.
Acid value á401ñ—
[NOTE—This test must be performed promptly after sampling to avoid oxidation of the test specimen.]Dissolve about 30g,accurately weighed,in 30mLof neutralized alcohol,add 1mLof phenolphthalein TS,and titrate with 0.01Nalcoholic potassium hydroxide VSto produce a permanent,faint pink color:the acid value so obtained is not more than 0.1.
Peroxide value á401ñ:
not more than 8.0,about 2g,accurately weighed,being used.
Limit of free ethylene oxide—
Acetaldehyde solution—
Prepare a solution of acetaldehyde in water containing a known concentration of about 10µg per mL.[NOTE—Prepare the Acetaldehyde solutionfresh just prior to use.]
Ethylene oxide stock solution—
[Caution—Ethylene oxide is toxic and flammable.Prepare these solutions in a well-ventilated fume hood,using great care.Protect both hands and face by wearing polyethylene protective gloves and an appropriate face mask.
][NOTE—Before using the polyethylene glycol 200in this test,remove any volatile components from it by placing 500mLof the polyethylene glycol 200in a 1000-mLround bottom flask,attaching the flask to a rotary evaporator,and evaporating at a temperature of 60 and at a pressure of 1.5kPa to 2.5kPa for 6hours.]Fill a chilled pressure bottle with liquid ethylene oxide,and store in a freezer when not in use.Use a small piece of polyethylene film to protect the liquid from contact with the rubber gasket.Tare a glass-stoppered conical flask,add about 50mLof polyethylene glycol 200,and reweigh the flask.Transfer about 5mLof the liquid ethylene oxide to a 100-mLbeaker chilled in a mixture of sodium chloride and wet ice (1:3).Using a gas-tight gas chromatographic syringe that has been previously cooled to -10
and at a pressure of 1.5kPa to 2.5kPa for 6hours.]Fill a chilled pressure bottle with liquid ethylene oxide,and store in a freezer when not in use.Use a small piece of polyethylene film to protect the liquid from contact with the rubber gasket.Tare a glass-stoppered conical flask,add about 50mLof polyethylene glycol 200,and reweigh the flask.Transfer about 5mLof the liquid ethylene oxide to a 100-mLbeaker chilled in a mixture of sodium chloride and wet ice (1:3).Using a gas-tight gas chromatographic syringe that has been previously cooled to -10 ,transfer about 300µL(corresponding to about 250mg)of liquid ethylene oxide to the polyethylene glycol 200,and swirl gently to mix.Replace the stopper,reweigh the flask,and determine the amount of ethylene oxide absorbed by weight difference.Adjust the weight of the mixture with polyethylene glycol 200to 100.0g,replace the stopper,and swirl gently to mix.This stock solution contains about 2.5mg of ethylene oxide per g.[NOTE—Prepare this stock solution fresh just prior to use,and store in a refrigerator.]
,transfer about 300µL(corresponding to about 250mg)of liquid ethylene oxide to the polyethylene glycol 200,and swirl gently to mix.Replace the stopper,reweigh the flask,and determine the amount of ethylene oxide absorbed by weight difference.Adjust the weight of the mixture with polyethylene glycol 200to 100.0g,replace the stopper,and swirl gently to mix.This stock solution contains about 2.5mg of ethylene oxide per g.[NOTE—Prepare this stock solution fresh just prior to use,and store in a refrigerator.]
Ethylene oxide standard solution A—
Tare a glass-stoppered conical flask,and chill it in a refrigerator.Add about 35mLof polyethylene glycol 200,and reweigh the flask.Using a gas-tight gas chromatographic syringe that has been chilled in a refrigerator,transfer about 1g of the chilled Ethylene oxide stock solution,accurately weighed,to the tared,conical flask.Adjust the weight of the solution with polyethylene glycol 200to 50.0g,replace the stopper,and swirl gently to mix.Transfer about 10g of this solution,accurately weighed,to a 50-mLvolumetric flask.Add 30mLof water,and mix.Dilute with water to volume,and mix to obtain a solution containing about 10µg of ethylene oxide per mL.[NOTE—Prepare this solution fresh just prior to use,and store in a refrigerator.]
Ethylene oxide standard solution B—
Transfer 10.0mLof Ethylene oxide standard solution Ato a 50-mLvolumetric flask,dilute with water to volume,and mix to obtain a solution containing about 2µg of ethylene oxide per mL.[NOTE—Prepare this solution fresh just prior to use,and store in a refrigerator.]
Standard solutions—
Transfer 0.5mLof Ethylene oxide standard solution Bto a 10-mLpressure headspace vial,add 0.1mLof Acetaldehyde solution and 0.1mLof water,seal the vial,and mix.Heat the mixture at 70 for 45minutes to obtain Standard solution A.Transfer about 1g of Diethylene Glycol Monoethyl Ether,accurately weighed,to a 10-mLpressure headspace vial,add 0.5mLof Ethylene oxide standard solution Band 0.5mLof water.Seal the vial,and mix.Heat the mixture at 70
for 45minutes to obtain Standard solution A.Transfer about 1g of Diethylene Glycol Monoethyl Ether,accurately weighed,to a 10-mLpressure headspace vial,add 0.5mLof Ethylene oxide standard solution Band 0.5mLof water.Seal the vial,and mix.Heat the mixture at 70 for 45minutes to obtain Standard solution B.
for 45minutes to obtain Standard solution B.
Test solution—
Transfer about 1g of Diethylene Glycol Monoethyl Ether,accurately weighed,to a 10-mLpressure headspace vial,add 1mLof water,seal the vial,and mix.Heat the mixture at 70 for 45minutes to obtain the Test solution.
for 45minutes to obtain the Test solution.
Chromatographic system
(see Chromatography á621ñ)—[NOTE—The use of a headspace apparatus that automatically transfers a measured amount of headspace is allowed.]The gas chromatograph is equipped with a flame-ionization detector,maintained at about 250 ,and a 0.32-mm ×30-m glass or quartz capillary column bonded with a 1.0-µm layer of phase G1.The injection port temperature is maintained at about 150
,and a 0.32-mm ×30-m glass or quartz capillary column bonded with a 1.0-µm layer of phase G1.The injection port temperature is maintained at about 150 .The column temperature is maintained at 50
.The column temperature is maintained at 50 for 5minutes after injection,then programmed to increase at the rate of 5
for 5minutes after injection,then programmed to increase at the rate of 5 per minute to 180
per minute to 180 ,then at the rate of 30
,then at the rate of 30 per minute to 230
per minute to 230 and to maintain this temperature for 5minutes.The carrier gas is helium flowing at a rate of about 1mLper minute.Chromatograph the gaseous phase of Standard solution A,and record the peak responses as directed for Procedure,adjusting the sensitivity of the system so that the peak heights of the two principal peaks in the chromatogram are not less than 15%of the full scale of the recorder:the relative retention times are about 0.94for acetaldehyde and 1.0for ethylene oxide;the resolution,R,between the acetaldehyde and ethylene oxide peaks is not less than 2.0;and the relative standard deviation for replicate injections is not more than 15%.
and to maintain this temperature for 5minutes.The carrier gas is helium flowing at a rate of about 1mLper minute.Chromatograph the gaseous phase of Standard solution A,and record the peak responses as directed for Procedure,adjusting the sensitivity of the system so that the peak heights of the two principal peaks in the chromatogram are not less than 15%of the full scale of the recorder:the relative retention times are about 0.94for acetaldehyde and 1.0for ethylene oxide;the resolution,R,between the acetaldehyde and ethylene oxide peaks is not less than 2.0;and the relative standard deviation for replicate injections is not more than 15%.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Using a heated,gas-tight gas chromatographic syringe,separately inject equal volumes (about 1mL)of the gaseous headspace of Standard solution Band the Test solutioninto the chromatograph,record the chromatograms,and measure the peak responses:the mean area of the ethylene oxide peak in the chromatogram obtained from the Test solutionis not greater than half the mean area of the corresponding peak in the chromatogram obtained from Standard solution B.Calculate the amount of ethylene oxide in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
rU/[(rSWU)-(rUWS)],
in which rUand rSare the ethylene oxide peak responses obtained from the Test solutionand Standard solution B,respectively;and WUand WSare the weights,in g,of Diethylene Glycol Monoethyl Ether taken to prepare the Test solutionand Standard solution B,respectively:not more than 1µg per g is found.
Limit of 2-methoxyethanol,2-ethoxyethanol,ethylene glycol,and diethylene glycol—
System suitability solution
and Chromatographic system—Proceed as directed in the Assay.
Procedure—
Proceed as directed in the Assay.Calculate the percentage of 2-methoxyethanol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri0/rs),
in which ri0is the peak response for 2-methoxyethanol;and rsis the sum of the responses of all the peaks:not more than 50µg per g of 2-methoxyethanol is found.Calculate the percentage of 2-ethoxyethanol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri1/rs),
in which ri1is the peak response for 2-ethoxyethanol:not more than 160µg per g of 2-ethoxyethanol is found.Calculate the percentage of ethylene glycol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri2/rs),
in which ri2is the peak response for ethylene glycol:not more than 620µg per g of ethylene glycol is found.Calculate the percentage of diethylene glycol in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(ri3/rs),
in which ri3is the peak response for diethylene glycol:not more than 150µg per g of diethylene glycol is found.
Assay—
System suitability solution—
Prepare a solution in methanol containing about 1mg each of 2-methoxyethanol,2-ethoxyethanol,ethylene glycol,diethylene glycol,and USP Diethylene Glycol Monoethyl Ether RSper mL.
Chromatographic system
(see Chromatography á621ñ)—The gas chromatograph is equipped with a flame-ionization detector,maintained at a temperature of about 275 ,and a 0.32-mm ×30-m fused-silica column bonded with a 1.0-µm layer of phase G46.The column temperature is programmed to be maintained at about 120
,and a 0.32-mm ×30-m fused-silica column bonded with a 1.0-µm layer of phase G46.The column temperature is programmed to be maintained at about 120 for 1minute,then to increase to 225
for 1minute,then to increase to 225 at a rate of 12
at a rate of 12 per minute,and to be maintained at 225
per minute,and to be maintained at 225 for 2minutes.The injection port temperature is maintained at about 250
for 2minutes.The injection port temperature is maintained at about 250 .Helium is used as the carrier gas at a flow rate of about 2.2mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.40for 2-methoxyethanol,0.43for 2-ethoxyethanol,0.50for ethylene glycol,0.93for diethylene glycol monoethyl ether,and 1.0for diethylene glycol;the resolution,R,between 2-ethoxyethanol and ethylene glycol is not less than 2.0;and the relative standard deviation for replicate injections is not more than 2.0%determined from diethylene glycol monoethyl ether.
.Helium is used as the carrier gas at a flow rate of about 2.2mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.40for 2-methoxyethanol,0.43for 2-ethoxyethanol,0.50for ethylene glycol,0.93for diethylene glycol monoethyl ether,and 1.0for diethylene glycol;the resolution,R,between 2-ethoxyethanol and ethylene glycol is not less than 2.0;and the relative standard deviation for replicate injections is not more than 2.0%determined from diethylene glycol monoethyl ether.
Procedure—
Inject a volume (about 0.5µL)of Diethylene Glycol Monoethyl Ether into the chromatograph,record the chromatogram,and measure the areas for the major peaks.Calculate the percentage of C6H14O3in the portion of Diethylene Glycol Monoethyl Ether taken by the formula:
100(A/B),
in which Ais the diethylene glycol monoethyl ether peak response;and Bis the sum of the responses of all the peaks in the chromatogram.
Auxiliary Information—
Staff Liaison:Daniel K.Bempong,Ph.D.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 2999
Phone Number:1-301-816-8143