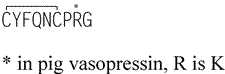Vasopressin
Vasopressin,8-L-arginine- [113-79-1].
C46H65N13O12S2 1056.22
Vasopressin,8-L-lysine- [50-57-7].
»Vasopressin is a polypeptide hormone having the properties of causing the contraction of vascular and other smooth muscles,and of antidiuresis.It is prepared by synthesis or obtained from the posterior lobe of the pituitary of healthy,domestic animals used for food by humans.Its vasopressor activity is not less than 300USP Vasopressin Units per mg.
Packaging and storage—
Preserve in tight containers,preferably of Type Iglass,in a refrigerator.
Microbial limits á61ñ—
The total bacterial count does not exceed 200cfu per g.For products of animal origin,it meets also the requirements of the tests for absence of Salmonellaspecies and Escherichia coli.
Identification—
A:
The retention time of the vasopressin peak in the chromatogram of the Assay preparationcorresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
B:
Bioidentity—About 18hours prior to the test,select a male rat weighing between 275g and 325g.Inject,subcutaneously,1mLper kg of body weight of a solution prepared by dissolving 50mg of phenoxybenzamine hydrochloride in 0.1mLof alcohol,acidifying with 1drop of hydrochloric acid,and diluting with saline TSto 5mL.On the day of the test,anesthetize the rat,using an anesthetic substance that favors the maintenance of a uniform blood pressure.Secure the animal,and cannulate the trachea for artificial respiration.Arrange to obtain a continuous record of the blood pressure from the carotid artery.Arrange for intravenous injections by means of a suitable cannula approximately 1mm in external diameter inserted into a femoral or jugular vein.Keep the animal warm during its preparation and during the test.Determine by trial the dose of the Standard preparationwhich,when injected intravenously at regular intervals of 12to 15minutes,will produce consistent blood pressure elevations of between 20and 70mm of mercury.Select the 2doses that would be in a ratio of approximately 2to 3,and prepare 2doses of the Test preparationthat correspond to the doses of the selected Standard preparations.Inject the rat with each dose in replicate of the Standard preparationand the Test preparationin a random fashion at regular intervals of 12to 15minutes,and record the blood pressures.The requirements of the test are met if the increase in blood pressure between the low dose and the high dose of the Standard preparationis comparable to that of the Test preparation.
Oxytocic activity (for product labeled of animal origin)—
Proceed as directed in the Assayunder Oxytocin,except that a suitable dilution of the USP Oxytocin RSwill contain approximately 1.2USP Oxytocin Units per mLof Standard preparation.The oxytocic activity of the Test preparationis not more than 1.2USP Oxytocin Units per mL.
Ordinary impurities—
The sum of the responses of impurities in the chromatogram of the Assay preparationobtained in the Assayis not more than 5%of the area of the vasopressin peak.
Assay—
Mobile phase—
Dissolve 6.6g of dibasic ammonium phosphate in about 950mLof water,and adjust with concentrated phosphoric acid to a pHof 3.0.Dilute with water to 1L,and mix.To 870mLof this solution add 130mLof acetonitrile,and mix.Filter under vacuum through a 0.45-µm nylon membrane.[NOTE—The retention time of the vasopressin peak is very sensitive to small changes in acetonitrile concentration in the Mobile phase.]
Diluent—
Dissolve 5.0g of chlorobutanol in 5.0mLof glacial acetic acid,add 5.0g of alcohol,1.1g of sodium acetate,and 1000mLof water,and mix.
Standard preparation—
Dissolve the entire contents of a vial of USP Vasopressin RSin a known volume of Diluent.[NOTE—The solution may be diluted as necessary to a working concentration range for the assay.]
Assay preparation—
Transfer about 10mg of Vasopressin,accurately weighed,to a 100-mLvolumetric flask.Dissolve in 0.25%glacial acetic acid,and dilute with the same solvent to volume.Mix,and pipet 5.0mLof this solution into a 100-mLvolumetric flask,and dilute with 0.25%glacial acetic acid to volume.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a variable wavelength detector set at 220mm and a 4.6-mm ×25-cm column that contains packing L1.The flow rate is 1.0mLper minute.The column is allowed to equilibrate for 1hour before making the first injection.Determine the suitability of the system (see System Suitabilityunder Chromatography á621ñ)as follows.Inject 20µLof the Standard preparationinto the equilibrated liquid chromatograph,allow about 60minutes for complete elution,and record the chromatogram as directed for Procedure:the retention time of the vasopressin peak is between 6and 9minutes,and is completely resolved from adjacent peaks;the resolution,R,between vasopressin and the nearest adjacent peak is not less than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%for vasopressin.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the potency of Vasopressin,in USP Vasopressin Units per mg,by the formula:
20C(rU/rS)(V/W),
in which Vis the volume of sample solution in which the sample was dissolved;and Wis the amount,in mg,of vasopressin dissolved in the sample solution;Cis the concentration in USP Vasopressin Units per mLin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Larry N.Callahan,Ph.D.,Scientist
Expert Committee:(BNT)Biotechnology and Natural Therapeutics/Diagnostics
USP28–NF23Page 2016
Pharmacopeial Forum:Volume No.29(6)Page 2004
Phone Number:1-301-816-8385