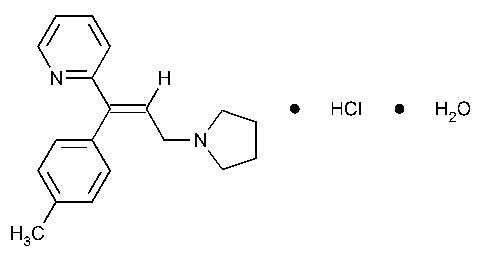
Triprolidine Hydrochloride
Pyridine,2-[1-(4-methylphenyl)-3-(1-pyrrolidinyl)-1-propenyl]-,monohydrochloride,monohydrate,(E)-.
(E)-2-[3-(1-Pyrrolidinyl)-1-p-tolylpropenyl]pyridine monohydrochloride monohydrate [6138-79-0].
Anhydrous 314.86 [550-70-9].
»Triprolidine Hydrochloride contains not less than 98.0percent and not more than 101.0percent of C19H22N2·HCl,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
USP Reference standards á11ñ—
USP Triprolidine Hydrochloride RS.USP Triprolidine Hydrochloride Z-isomer RS.
Identification—
A:
Infrared Absorption á197Kñ.
Solution:
10µg per mL.
Medium:
0.1Nhydrochloric acid.
Absorptivities at 290nm,calculated on the anhydrous basis,do not differ by more than 3.0%.
C:
Asolution of it responds to the tests for Chloride á191ñ.
Water,Method Iá921ñ:
between 4.0%and 6.0%.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.002%.
Chromatographic purity—
Standard preparations—
Dissolve USP Triprolidine Hydrochloride RSin chloroform,and mix to obtain a solution having a known concentration of 1.0mg per mL.Dilute quantitatively with chloroform to obtain four diluted Standard preparations(A,B,C,and D)having the following compositions:
| Standard preparation | Dilution | Concentration (µg RSper mL) |
Percentage (%, for comparison with test specimen) |
| A | (1in 5) | 200 | 2.0 |
| B | (15in 100) | 150 | 1.5 |
| C | (1in 10) | 100 | 1.0 |
| D | (5in 100) | 50 | 0.5 |
Standard Z-isomer preparations—
Proceed as directed for Standard preparations,using USP Triprolidine Hydrochloride Z-isomer RSto obtain four diluted Standard preparationshaving the same compositions as in the table shown therein.
Test preparation—
Dissolve an accurately weighed quantity of Triprolidine Hydrochloride in chloroform to obtain a solution containing 10mg per mL.
Procedure—
Apply separately 5µLof the Test preparationand 5µLof each of the eight diluted Standard to a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Position the plate in a chromatographic chamber,and develop the chromatograms,protected from light,in a solvent system consisting of a mixture of chloroform and diethylamine (95:5)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and allow the solvent to evaporate.Examine the plate under long-and short-wavelength UVlight.Compare the intensities of any secondary spots observed in the chromatogram of the Test preparationwith those of the principal spots in the chromatograms of the Standard preparations:the intensity of the Z-isomer triprolidine hydrochloride spot (RFvalue about 1.2relative to the RFvalue for triprolidine hydrochloride)obtained from the Test preparationcorresponds to not more than 2.0%,and the sum of the intensities of all secondary spots obtained from the Test preparationcorresponds to not more than 3.0%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Assay—
Dissolve about 400mg of Triprolidine Hydrochloride,accurately weighed,in 80mLof glacial acetic acid,warming,if necessary,to effect solution.Add 15mLof mercuric acetate TS,and titrate with 0.1Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 15.74mg of C19H22N2·HCl.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 1991
Phone Number:1-301-816-8379