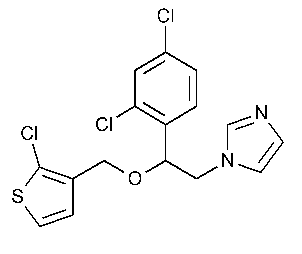
Tioconazole
1H-Imidazole,1-[2-[(2-chloro-3-thienyl)methoxy]-2-(2,4-dichlorophenyl)ethyl]-.
1-[2,4-Dichloro-[b-(2-chloro-3-thenyl)-oxy]phenethyl]imidazole [65899-73-2].
»Tioconazole contains not less than 97.0percent and not more than 103.0percent of C16H13Cl3N2OS.
Packaging and storage—
Preserve in tight containers.
USP Reference standards á11ñ—
USP Tioconazole RS.USP Tioconazole Related Compound A RS.USP Tioconazole Related Compound B RS.USP Tioconazole Related Compound C RS.
Identification—
A:
Infrared Absorption á197Mñ.
B:
Visualizing solution—Dissolve 0.85g of bismuth subnitrate in 10mLof glacial acetic acid,dilute with water to 50mL,and mix.Mix 10mLof this solution,50mLof potassium iodide solution (2in 25),and 20mLof glacial acetic acid,dilute with water to 100mL,and mix.
Procedure—
Prepare a test solution by dissolving 50mg of Tioconazole in 1mLof methanol.Separately apply 10µLof the test solution and 10µLof a Standard solution of USP Tioconazole RS,similarly prepared,to a thin-layer chromatographic plate (see Chromatography á621ñ),coated with a 0.25-mm layer of chromatographic silica gel mixture.Allow the spots to dry,and develop the chromatogram using a solvent system consisting of a mixture of chloroform,methanol,and glacial acetic acid (40:5:1)until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the developing chamber,mark the solvent front,and locate the spots on the plate by viewing under short-and long-wavelength UVlight after drying the plate at 80 for 5minutes.Spray the plate with Visualizing solution,air-dry for 2minutes,and overspray with sodium nitrite solution (1in 20).Air-dry the plate for 5minutes,and examine it for brown spots on a pale yellow background:the RFvalue of the principal spot from the test solution corresponds to that obtained from the Standard solution.
for 5minutes.Spray the plate with Visualizing solution,air-dry for 2minutes,and overspray with sodium nitrite solution (1in 20).Air-dry the plate for 5minutes,and examine it for brown spots on a pale yellow background:the RFvalue of the principal spot from the test solution corresponds to that obtained from the Standard solution.
Water,Method Iá921ñ:
not more than 0.5%.
Residue on ignition á281ñ:
not more than 0.2%.
Chloride á221ñ—
A0.7-g portion dissolved in methanol shows no more chloride than corresponds to 0.50mLof 0.020Nhydrochloric acid (0.05%).
Heavy metals,Method IIá231ñ:
0.005%.
Related compounds—
Standard preparation—
Transfer about 1mg each,accurately weighed,of USP Tioconazole Related Compound A RS,USP Tioconazole Related Compound B RS,and USP Tioconazole Related Compound C RSto a 25-mLflask.Add 15.0mLof methanol,and shake until the contents are completely dissolved.
Test preparation—
Transfer about 100mg of Tioconazole,accurately weighed,to a 25-mLflask,add 15.0mLof methanol,and shake until the substance is completely dissolved.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Test preparationinto the chromatograph,record the chromatograms,and measure the responses for the peaks.Calculate,in turn,the percentages of 1-[2,4-dichloro-b-[(3-thenyl)-oxy]phenethyl]imidazole hydrochloride (tioconazole related compound A),1-[2,4-dichloro-b-[(2,5-dichloro-3-thenyl)-oxy]phenethyl]imidazole hydrochloride (tioconazole related compound B),and 1-[2,4-dichloro-b-[(5-bromo-2-chloro-3-thenyl)-oxy]phenethyl]imidazole hydrochloride (tioconazole related compound C)in the portion of Tioconazole taken by the same formula:
100(WI/WU)(rU/rS),
in which WIis the weight,in mg,of the respective USP Reference Standard taken to prepare the Standard preparation,WUis the weight,in mg,of Tioconazole taken to prepare the Test preparation,and rUand rSare the peak responses at corresponding retention times,obtained from the Test preparationand the Standard preparation,respectively.The limit of each related compound is 1.0%.
Assay—
Mobile phase—
[NOTE—Prepare the Mobile phasefresh daily.]
Mix 440mLof acetonitrile,400mLof methanol,and 280mLof water.Degas the solution.Add 2.0mLof ammonium hydroxide,and mix.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Tioconazole RSin methanol,and dilute quantitatively and stepwise,if necessary,with methanol to obtain a solution having a known concentration of about 200µg per mL.
Assay preparation—
Transfer about 100mg of Tioconazole,accurately weighed,to a 100-mLvolumetric flask,dissolve in methanol,dilute with methanol to volume,and mix.Transfer 10.0mLof the resulting solution to a 50-mLvolumetric flask,dilute with methanol to volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 219-nm detector,a 4-mm ×10-cm precolumn that contains packing L4,installed between the pump and the injector,and a 5-mm ×25-cm analytical column that contains packing L1.[NOTE—Replace the precolumn daily.]The flow rate is adjusted to obtain a retention time of between 12and 17minutes for tioconazole.Chromatograph the Standard preparation,and record the peak responses as directed under Procedure.The column efficiency determined from the analyte peak is not less than 1000theoretical plates,the tailing factor for the analyte peak is not more than 2.0,and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C16H13Cl3N2OSin the Tioconazole taken by the formula:
(0.5C)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Tioconazole RS,calculated on the anhydrous basis,in the Standard preparation,and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1936
Phone Number:1-301-816-8394