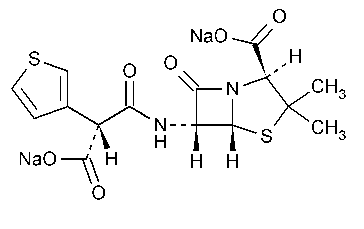
Ticarcillin Disodium
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,6-(carboxy-3-thienylacetyl)amino-3,3-dimethyl-7-oxo-,disodium salt,[2S-2a,5a,6b(S*)]-.
N-(2-Carboxy-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]-hept-6-yl)-3-thiophenemalonamic acid disodium salt [4697-14-7].
»Ticarcillin Disodium has a potency equivalent to not less than 800µg of ticarcillin (C15H16N2O6S2)per mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
Solution:
20µg per mL.Use the solution prepared as directed in the test for Ticarcillin content,recording the spectrum between 200and 300nm.
B:
Asolution (1in 20)responds to the tests for Sodium á191ñ.
Specific rotation á781Sñ:
between +172 and +187
and +187 .
.
Test solution:
10mg per mL,in water.
pHá791ñ:
between 6.0and 8.0,in a solution containing 10mg of ticarcillin per mL.
Water,Method Iá921ñ:
not more than 6.0%.
Dimethylaniline á223ñ:
meets the requirement.
Ticarcillin content—
Transfer about 40mg,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.Transfer 5.0mLof this solution to another 100-mLvolumetric flask,dilute with 0.1Nmethanolic hydrochloric acid (0.8mLof hydrochloric acid diluted with methanol to 100mL)to volume,and mix.Concomitantly determine the absorbances of this test solution with a similarly prepared Standard solution of USP Ticarcillin Monosodium Monohydrate RS,at the wavelength of maximum absorbance at about 230nm,using a reagent blank.Calculate the percentage of ticarcillin (C15H16N2O6S2)taken by the formula:
P(WS/WU)(AU/AS),
in which Pis the percentage content of ticarcillin in USP Ticarcillin Monosodium Monohydrate RS;WSand WUare the amounts of USP Ticarcillin Monosodium Monohydrate RSand Ticarcillin Disodium taken,respectively;and AUand ASare the absorbances of the test solution and the Standard solution,respectively:between 80.0%and 94.0%,calculated on the anhydrous basis,is found.
Other requirements—
Where the label states that Ticarcillin Disodium is sterile,it meets the requirements for Sterility Tests á71ñand for Bacterial endotoxinsunder Ticarcillin for Injection.Where the label states that Ticarcillin Disodium must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Bacterial endotoxinsunder Ticarcillin for Injection.
Assay—
pH4.3sodium phosphate buffer,Mobile phase,pH6.4sodium phosphate buffer,Standard preparation,Resolution solution,and Chromatographic system—
Proceed as directed in the Assayunder Ticarcillin Monosodium.
Assay preparation—
Transfer about 50mg of Ticarcillin Disodium,accurately weighed,to a 50-mLvolumetric flask,add pH6.4sodium phosphate bufferto volume,and mix.
Procedure—
Proceed as directed for Procedurein the Assayunder Ticarcillin Monosodium.Calculate the quantity,in µg,of ticarcillin (C15H16N2O6S2)per mg of the Ticarcillin Disodium taken by the formula:
50(CP/M)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Ticarcillin Monosodium Monohydrate RSin the Standard preparation;Pis the designated potency,in µg of ticarcillin per mg,of USP Ticarcillin Monosodium Monohydrate RS;Mis the quantity,in mg,of Ticarcillin Disodium taken to prepare the Assay preparation;and rUand rSare the ticarcillin peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1927
Phone Number:1-301-816-8335