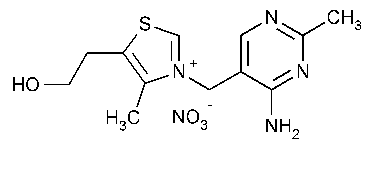
Thiamine Mononitrate
Thiazolium,3-[(4-amino-2-methyl-5-pyrimidinyl)methyl]-5-(2-hydroxyethyl)-4-methyl-,nitrate (salt).
Thiamine nitrate (salt) [532-43-4].
»Thiamine Mononitrate contains not less than 98.0percent and not more than 102.0percent of C12H17N5O4S,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
To 2mLof a solution (1in 50)add 2mLof sulfuric acid,cool,and superimpose 2mLof ferrous sulfate TS:a brown ring is produced at the junction of the two liquids.
B:
Dissolve about 5mg in a mixture of 1mLof lead acetate TSand 1mLof 2.5Nsodium hydroxide:a yellow color is produced.Heat the mixture for several minutes on a steam bath:the color changes to brown,and,on standing,a precipitate of lead sulfide separates.
D:
Dissolve about 5mg in 5mLof 0.5Nsodium hydroxide,and proceed as directed in Identificationtest Bunder Thiamine Hydrochloride Injection,beginning with “then add 0.5mLof potassium ferricyanide TS”:the specified reaction is observed.
pHá791ñ:
between 6.0and 7.5,in a solution (1in 50).
Loss on drying á731ñ—
Dry about 500mg,accurately weighed,at 105 for 2hours:it loses not more than 1.0%of its weight.
for 2hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.2%.
Chloride á221ñ—
A500-mg portion shows no more chloride than corresponds to 0.40mLof 0.020Nhydrochloric acid (0.06%).
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Chromatographic purity—
Solution A
,Solution B,and Mobile phase—Prepare as directed in the Assayunder Thiamine Hydrochloride.
Test solution—
Dissolve quantitatively an accurately weighed quantity of Thiamine Mononitrate in Mobile phaseto obtain a solution having a concentration of about 1.0mg per mL.
Chromatographic system—
Proceed as directed in the test for Chromatographic purityunder Thiamine Hydrochloride.
Procedure—
Proceed as directed in the test for Chromatographic purityunder Thiamine Hydrochloride:the total of the responses of all secondary peaks is not greater than 1.0%of the total of the responses of all of the peaks.
Assay—
Solution A
,Solution B,Mobile phase,Internal standard solution,Standard preparation,and Chromatographic system—Proceed as directed in the Assayunder Thiamine Hydrochloride.
Assay preparation—
Transfer an accurately weighed quantity of about 200mg of Thiamine Mononitrate to a 100-mLvolumetric flask,dissolve in Mobile phase,dilute with Mobile phaseto volume,and mix.Transfer 10.0mLof this solution to a 50-mLvolumetric flask,add 5.0mLof Internal standard solution,dilute with Mobile phaseto volume,and mix.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the areas of the major peaks.Calculate the quantity,in mg,of C12H17N5O4Sin the Thiamine Mononitrate taken by the formula:
(327.36/337.27)0.5C(RU/RS),
in which 327.36and 337.27are the molecular weights of thiamine mononitrate and thiamine hydrochloride,respectively,Cis the concentration,in µg per mL,of USP Thiamine Hydrochloride RSin the Standard preparation,and RUand RSare the ratios of the peak areas of thiamine to methylbenzoate obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(DSN)Dietary Supplements:Non-Botanicals
USP28–NF23Page 1908
Phone Number:1-301-816-8389