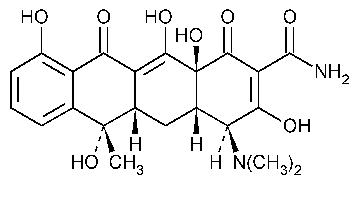
Tetracycline
2-Naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-,[4S-(4a,4aa,5aa,6b,12aa)]-.
(4S,4aS,5aS,12aS)-4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,6,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacene-carboxamide [60-54-8].
Trihydrate 498.49 [6416-04-2].
»Tetracycline has a potency equivalent to not less than 975µg of tetracycline hydrochloride (C22H24N2O8·HCl)per mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Labeling—
Label it to indicate that it is to be used in the manufacture of nonparenteral drugs only.
USP Reference standards á11ñ—
USP Tetracycline Hydrochloride RS.USP4-Epianhydrotetracycline Hydrochloride RS.
Identification—
A:
Ultraviolet Absorption á197Uñ—
Solution:
20µg per mL.
Medium:
0.25Nsodium hydroxide.
Absorptivity 6minutes after preparation,calculated on the anhydrous basis,at 380nm is between 104.5%and 111.95%of that of USP Tetracycline Hydrochloric RS,the potency of the Reference Standard being taken into account.
B:
The chromatogram of the Assay obtained as directed in the Assayexhibits a major peak for tetracycline,the retention time of which corresponds to that exhibited in the chromatogram of the Standard preparationobtained as directed in the Assay.
C:
To 0.5mg add 2mLof sulfuric acid:a purplish red color is produced.Add the solution to 1mLof water:the color becomes yellow.
D:
Prepare a Test Solutionin methanol containing the equivalent of 1mg of tetracycline hydrochloride per mL,and proceed as directed for Method IIunder Identification—Tetracyclines á193ñ.
Specific rotation á781Sñ:
between -260 and -280
and -280 ,calculated on the anhydrous basis.
,calculated on the anhydrous basis.
Test solution:
5mg per mL,in 0.1Nhydrochloric acid.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 3.0and 7.0,in an aqueous suspension containing 10mg per mL.
Water,Method Iá921ñ:
not more than 13.0%.
Heavy metals,Method IIá231ñ:
0.005%.
Limit of 4-epianhydrotetracycline—
Using the Diluting solvent,Chromatographic system,and Procedureset forth in the Assay,chromatograph a Standard solution prepared by dissolving an accurately weighed quantity of USP4-Epianhydrotetracycline Hydrochloride RSin Diluting solventto obtain a solution having a known concentration of about 10µg per mL.Using the chromatogram so obtained and the chromatogram of the Assay preparationobtained as directed in the Assay,calculate the percentage of 4-epianhydrotetracycline in the Tetracycline taken by the formula:
10(CE/W)(rU/rS),
in which CEis the concentration,in µg per mL,of USP4-Epianhydrotetracycline Hydrochloride RSin the Standard solution,Wis the weight,in mg,of Tetracycline taken to prepare the Assay preparation,and rUand rSare the 4-epianhydrotetracycline peak responses obtained from the Assay preparationand the Standard solution,respectively:not more than 2.0%is found.
Assay—
Diluting solvent,Mobile phase,Standard preparation,Resolution solution,and Chromatographic system—
Prepare as directed in the Assayunder Tetracycline Hydrochloride.
Assay preparation—
Transfer about 45mg of Tetracycline,accurately weighed,to a 100-mLvolumetric flask,dissolve in Diluting solvent,dilute with the same solvent to volume,and mix.
Procedure—
Proceed as directed in the Assayunder Tetracycline Hydrochloride.Calculate the quantity,in µg,of tetracycline hydrochloride (C22H24N2O8·HCl)equivalent in each mg of Tetracycline taken by the formula:
100(CP/W)(rU/rS),
in which Wis the weight,in mg,of Tetracycline taken to prepare the Assay preparation,and the other terms are as defined therein.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1884
Phone Number:1-301-816-8335