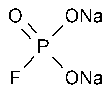
Sodium Monofluorophosphate
»Sodium Monofluorophosphate contains not less than 91.7percent and not more than 100.5percent of Na2PFO3,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Place about 1g in a platinum crucible in a well-ventilated hood,and add 15mLof sulfuric acid.Cover the crucible with a piece of clear,polished glass,and heat on a steam bath for 1hour.Remove the glass cover,rinse it in water,and dry:the glass surface exposed to vapors from the crucible is etched.
B:
Asolution (1in 10)with silver nitrate TSyields a white precipitate,which is soluble in diluted nitric acid and in dilute ammonium hydroxide (1in 2).
C:
Asolution responds to the tests for Sodium á191ñ.
pHá791ñ:
between 6.5and 8.0,in a solution (1in 50).
Loss on drying á731ñ—
Dry it at 105 to constant weight:it loses not more than 0.2%of its weight.
to constant weight:it loses not more than 0.2%of its weight.
Arsenic,Method Iá211ñ:
3ppm.
Heavy metals,Method Iá231ñ—
Dissolve 400mg in 25mLof water:the limit is 0.005%.
Limit of fluoride ion—
[NOTE—Use plasticware throughout this test.]
Buffer solution—
To 55g of sodium chloride in a 1000-mLvolumetric flask add 500mg of sodium citrate,255g of sodium acetate,and 300mLof water.Shake to dissolve,and cautiously add 115mLof glacial acetic acid with mixing.Cool to room temperature,add 300mLof isopropyl alcohol,dilute with water to volume,and mix:the pHof this solution is between 5.0and 5.5.
Standard stock solution—
Dissolve an accurately weighed quantity of USP Sodium Fluoride RSquantitatively in water to obtain a solution containing 1105µg per mL.Each mLof this solution contains 500µg of fluoride ion.Store in a tightly closed,plastic container.
Standard preparations—
To four 100-mLvolumetric flasks transfer,respectively,2.0-,4.0-,10.0-,and 20.0-mLportions of the Standard stock solution,dilute each with Buffer solutionto volume,and mix to obtain solutions having fluoride ion concentrations of 10,20,50,and 100µg per mL,respectively.
Test preparation—
Transfer about 1.8g,accurately weighed,to a 100-mLvolumetric flask,add water to dissolve,dilute with water to volume,and mix.Transfer 20.0mLof this solution to a second 100-mLvolumetric flask,dilute with Buffer solutionto volume,and mix.
Procedure—
Concomitantly measure the potential (see pHá791ñ),in mV,of the Standard preparationsand of the Test preparationwith a pHmeter capable of a minimum reproducibility of ±0.2mV,equipped with a glass-sleeved calomel-fluoride specific-ion electrode system.[NOTE—When taking measurements,immerse the electrodes in the solution,which has been transferred to a 150-mLplastic beaker containing a plastic-coated stirring bar.Allow to stir on a magnetic stirrer having an insulated top until equilibrium is attained (1to 2minutes),and record the potential.Rinse and dry the electrodes between measurements,taking care to avoid damaging the crystal of the specific-ion electrode.]Plot the logarithm of the fluoride-ion concentrations,in µg per mL,of each Standard preparationversus potential,in mV.From the measured potential of the Test preparationand the standard curve,determine the concentration,in µg per mL,of fluoride ion in the Test preparation:not more than 1.2%is found.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Monochloroacetate buffer—
Dissolve 189g of monochloroacetic acid and 55g of sodium hydroxide in about 1500mLof water.Cool,dilute with water to 2000mL,and mix.
0.025M Thorium nitrate—
Dissolve 13.8g of thorium nitrate in about 800mLof water,and filter the solution into a 1000-mLvolumetric flask.Dilute with water to volume,and mix.Standardize this solution as follows.Transfer 20.0mLof Standard stock solution,prepared as directed in the test for Limit of fluoride ion,to a 150-mLbeaker containing 50mLof water.Add 3drops of sodium alizarinsulfonate TS,mix,and adjust the acidity by the careful addition,successively,of sodium hydroxide solution (1in 50)and dilute hydrochloric acid (1in 160),until the pink color has just been discharged.Add 1mLof Monochloroacetate buffer,and titrate with 0.025M Thorium nitrateto a permanent pink color.Calculate the molarity of the thorium nitrate titrant taken by the equation:
M=(S)/[(4)(41.99V)],
in which Sis the weight,in mg,of USP Sodium Fluoride RSin the portion of Standard stock solutiontaken,41.99is the molecular weight of sodium fluoride,and Vis the volume,in mL,of titrant consumed.
Procedure—
Transfer about 1.8g of Sodium Monofluorophosphate,accurately weighed,to a 100-mLvolumetric flask.Add about 50mLof water,mix to effect solution,dilute with water to volume,and mix.Transfer 20.0mLof this solution to the reaction flask of a suitable fluoride steam distilling apparatus containing 10lime-glass beads measuring 5mm in diameter and 70mLof dilute sulfuric acid (1in 2).Distill,by passing steam through the solution in the reaction flask and applying heat to the flask,collecting the distillate in a 250-mLvolumetric flask.Control the temperature of the solution in the reaction flask so that it does not exceed 140 .Change receivers when the flask is filled to volume with distillate,and continue distilling into a 400-mLbeaker until an additional 150mLto 200mLof distillate tailing has been collected.Mix the solution in the 250-mLvolumetric flask,and transfer 50.0mLto a 150-mLbeaker.To the 150-mLbeaker add 50mLof water and 3drops of sodium alizarinsulfonate TS,and mix.Adjust the acidity of this solution by the careful addition,successively,of sodium hydroxide solution (1in 50)and dilute hydrochloric acid (1in 160),until the pink color has just been discharged.Add 1mLof Monochloroacetate buffer,titrate with 0.025M Thorium nitrateto a permanent pink color,and record the volume,in mL,of titrant consumed as VA.Add 3drops of sodium alizarinsulfonate TSto the distillation tail in the 400-mLbeaker,proceed as directed previously with the adjustment of the acidity,the addition of Monochloroacetate buffer,and the titration with 0.025M Thorium nitrate,and record the volume,in mL,of titrant consumed as VB.Calculate the percentage of Na2PFO3in the Sodium Monofluorophosphate taken by the formula:
.Change receivers when the flask is filled to volume with distillate,and continue distilling into a 400-mLbeaker until an additional 150mLto 200mLof distillate tailing has been collected.Mix the solution in the 250-mLvolumetric flask,and transfer 50.0mLto a 150-mLbeaker.To the 150-mLbeaker add 50mLof water and 3drops of sodium alizarinsulfonate TS,and mix.Adjust the acidity of this solution by the careful addition,successively,of sodium hydroxide solution (1in 50)and dilute hydrochloric acid (1in 160),until the pink color has just been discharged.Add 1mLof Monochloroacetate buffer,titrate with 0.025M Thorium nitrateto a permanent pink color,and record the volume,in mL,of titrant consumed as VA.Add 3drops of sodium alizarinsulfonate TSto the distillation tail in the 400-mLbeaker,proceed as directed previously with the adjustment of the acidity,the addition of Monochloroacetate buffer,and the titration with 0.025M Thorium nitrate,and record the volume,in mL,of titrant consumed as VB.Calculate the percentage of Na2PFO3in the Sodium Monofluorophosphate taken by the formula:
[(2)(143.95)(5VA+VB)(M)/(W)]-(143.95/18.9984)(F),
in which Mis the molarity of 0.025M Thorium nitrate;Wis the weight,in g,of the Sodium Monofluorophosphate taken;Fis the percentage of fluoride ions determined as directed in the test for Limit of fluoride ion;143.95is the molecular weight of sodium monofluorophosphate;18.9984is the atomic weight of fluorine;and the other terms are as defined therein.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 1788
Phone Number:1-301-816-8251