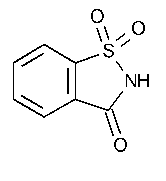
Saccharin
1,2-Benzisothiazol-3(2H)-one,1,1-dioxide.
1,2-Benzisothiazolin-3-one 1,1-dioxide [81-07-2].
»Saccharin contains not less than 98.0percent and not more than 101.0percent of C7H5NO3S,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Dissolve about 100mg in 5mLof sodium hydroxide solution (1in 20),evaporate the solution to dryness,and gently fuse the residue over a small flame until it no longer evolves ammonia.Allow the residue to cool,dissolve it in 20mLof water,neutralize the solution with 3Nhydrochloric acid,and filter:the addition of a drop of ferric chloride TSto the filtrate produces a violet color.
B:
Mix 20mg with 40mg of resorcinol,add 10drops of sulfuric acid,and heat the mixture in a suitable liquid bath at 200 for 3minutes.Allow it to cool,and add 10mLof water and an excess of 1Nsodium hydroxide:a fluorescent green liquid results.
for 3minutes.Allow it to cool,and add 10mLof water and an excess of 1Nsodium hydroxide:a fluorescent green liquid results.
Loss on drying á731ñ—
Dry it at 105 for 2hours:it loses not more than 1.0%of its weight.
for 2hours:it loses not more than 1.0%of its weight.
Readily carbonizable substances á271ñ—
Dissolve 200mg in 5mLof sulfuric acid TS,and keep at a temperature of 48 to 50
to 50 for 10minutes:the solution has no more color than Matching Fluid A.
for 10minutes:the solution has no more color than Matching Fluid A.
Residue on ignition á281ñ:
not more than 0.2%.
Toluenesulfonamides—
Internal standard solution—
Place 10mg of n-tricosane in a 10-mLvolumetric flask,dissolve in n-heptane,dilute with n-heptane to volume,and mix.
Standard stock solution—
Transfer 20mg each,accurately weighed,of USPo-Toluenesulfonamide RSand of USPp-Toluenesulfonamide RSto a 10-mLvolumetric flask,dissolve in methylene chloride,dilute with methylene chloride to volume,and mix.
Standard preparations—
Transfer 100,150,200,and 250µL,respectively,of Standard stock solutionto each of four 10-mLvolumetric flasks.Add 250µL,accurately measured,of Internal standard solutionto each flask,dilute each with methylene chloride to volume,and mix.These preparations contain,in each mL,25µg of n-tricosane and,respectively,20,30,40,and 50µg of each toluenesulfonamide isomer.
Test preparation—
Prepare as directed under Column Partition Chromatography(see Chromatography á621ñ),employing a chromatographic tube fitted with a porous glass disk in its base,a plastic stopcock on the delivery tube,and a reservoir at the top.Add a mixture consisting of 12g of Solid Supportand a solution of 2.0g,accurately weighed,of Saccharin with 12mLof filtered sodium bicarbonate solution (1in 11).Add about 200mg of sodium bicarbonate to effect complete solution of the saccharin.Pack the contents of the tube by tapping the column on a padded surface,and then by tamping firmly from the top.Place 100mLof methylene chloride in the reservoir,and adjust the stopcock so that 50mLof eluate is collected in 20to 30minutes.To the eluate add 25µLof Internal standard solution,mix,and concentrate the solution,by suitable means,to a volume of 1.0mL.
Chromatographic system (see Chromatography á621ñ)—
Under typical conditions,the instrument is equipped with a flame-ionization detector,and contains a 13.2-mm.×8-m glass column packed with 10%liquid phase G3on 100-to 120-mesh support S1AB,utilizing a glass-lined sample introduction system or on-column injection.The injector port,column,and detector block are maintained at temperatures of about 225 ,210
,210 ,and 250
,and 250 ,respectively,and dry helium is used as the carrier gas at a flow rate of about 30mLper minute.
,respectively,and dry helium is used as the carrier gas at a flow rate of about 30mLper minute.
Procedure—
Inject portions (about 2.5µL)of the Standard preparations,successively,into a gas chromatograph,and record each chromatogram so as to obtain at least 50%of maximum recorder response.Measure the areas under the first (o-toluenesulfonamide),second (p-toluenesulfonamide),and third (n-tricosane)peaks,and for each chromatogram record the values as Ao,Ap,and AN,respectively.Calculate the ratios Roand Rptaken by the equations:
Ro=Ao/ANand Rp=Ap/AN,
and prepare standard curves by plotting the concentrations,in µg per mL,of USPo-Toluenesulfonamide RSand of USPp-Toluenesulfonamide RSin the Standard preparationsversus Roand Rp,respectively.[NOTE—Relative retention times are,approximately,0.39for o-toluenesulfonamide,0.46for p-toluenesulfonamide,and 1.0for n-tricosane.]Similarly inject a portion (about 2.5µL)of the Test preparation,and record the chromatogram.Measure the areas under the first (o-toluenesulfonamide),second (p-toluenesulfonamide),and third (n-tricosane)peaks,and record the values as ao,ap,and aN,respectively.Calculate the ratios roand rptaken by the equations:
ro=ao/aNand rp=ap/aN,
and,from the standard curve,determine the concentration,in µg per mL,of each toluenesulfonamide isomer in the Test preparation:the total amount of toluenesulfonamides in the specimen taken is not more than 0.0025%.
Selenium á291ñ:
0.003%,a 100-mg specimen,mixed with 100mg of magnesium oxide,being used.
Heavy metals,Method IIá231ñ:
0.001%.
Benzoic and salicylic acids—
To 10mLof a hot,saturated solution of it add ferric chloride TS,dropwise:no precipitate or violet color appears in the liquid.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Accurately weigh about 500mg of Saccharin,dissolve in 40mLof alcohol,add 40mLof water,mix,add phenolphthalein TS,and titrate with 0.1Nsodium hydroxide VS.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nsodium hydroxide is equivalent to 18.32mg of C7H5NO3S.
Auxiliary Information—
Staff Liaison:Justin Lane,B.S.,Scientific Associate
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 3070
Pharmacopeial Forum:Volume No.30(4)Page 1450
Phone Number:1-301-816-8323