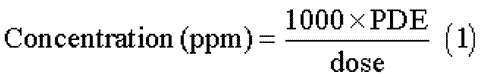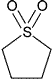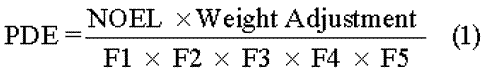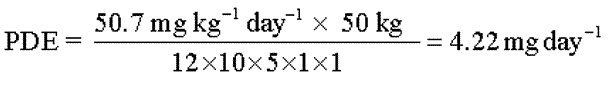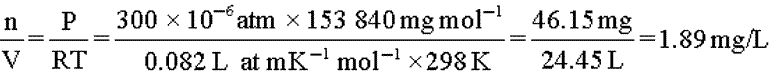á467ñORGANIC VOLATILE IMPURITIES
RESIDUAL SOLVENTS LIMITS
For pharmacopeial purposes,residual solvents in pharmaceuticals are defined as organic volatile chemicals that are used or produced in the manufacture of drug substances or excipients,or in the preparation of drug products.The residual solvents are not completely removed by practical manufacturing techniques.Appropriate selection of the solvent for the synthesis of a drug substance or an excipient may enhance the yield,or determine characteristics such as crystal form,purity,and solubility.Therefore,the solvent may sometimes be a critical element in the synthetic process.This General Chapter does not address solvents deliberately used as excipients nor does it address solvates.However,the content of solvents in such products should be evaluated and justified.
Because residual solvents do not provide therapeutic benefit,they should be removed,to the extent possible,to meet ingredient and product specifications,good manufacturing practices,or other quality-based requirements.Drug products should contain no higher levels of residual solvents than can be supported by safety data.Solvents that are known to cause unacceptable toxicities (Class 1,Table 1)should be avoided in the production of drug substances,excipients,or drug products unless their use can be strongly justified in a risk-benefit assessment.Solvents associated with less severe toxicity (Class 2,Table 2)should be limited in order to protect patients from potential adverse effects.Ideally,less toxic solvents (Class 3,Table 3)should be used where practical.The complete list of solvents included in this General Chapter is given in Appendix 1.These tables and the list are not exhaustive.Where other solvents have been used,based on approval by the competent regulatory authority,such solvents may be added to the tables and list.
Testing of drug substances,excipients,and drug products for residual solvents should be performed when production or purification processes are known to result in the presence of such residual solvents.It is only necessary to test for residual solvents that are used or produced in the manufacture or purification processes.
Although manufacturers may choose to test the drug product,a cumulative procedure may be used to calculate the residual solvent levels in the product from the levels in its ingredients.If the calculation results in a level equal to or below that recommended in this General Chapter,no testing of the drug product for residual solvents needs to be considered.If,however,the calculated levels are above the recommended level,the drug product should be tested to ascertain whether the formulation process has reduced the relevant solvent levels to within acceptable amounts.Adrug product should also be tested if a residual solvent is used during its manufacture.
See Appendix 2for additional background information related to residual solvents.
CLASSIFICATION OF RESIDUAL SOLVENTS BY RISK ASSESSMENT
The term “tolerable daily intake”(TDI)is used by the International Program on Chemical Safety (IPCS)to describe exposure limits of toxic chemicals and the term “acceptable daily intake”(ADI)is used by the World Health Organization (WHO)and other national and international health authorities and institutes.The term “permitted daily exposure”(PDE)is defined as a pharmaceutically acceptable intake of residual solvents to avoid confusion of differing values for ADIs of the same substance.
Residual solvents specified in this General Chapter are listed in Appendix 1by common names and structures.They were evaluated for their possible risk to human health and placed into one of three classes as follows:
| Class 1 | Residual Solvents:Solvents to be Avoided Known human carcinogens Strongly suspected human carcinogens Environmental hazards |
| Class 2 | Residual Solvents:Solvents to be Limited Nongenotoxic animal carcinogens or possible causative agents of other irreversible toxicity,such as neurotoxicity or teratoge nicity. Solvents suspected of other significant but rever- sible toxicities. |
| Class 3 | Residual Solvents:Solvents with Low Toxic Po- tential Solvents with low toxic potential to humans;no health-based exposure limit is needed. [NOTE—Class 3residual solvents may have PDEs of up to 50mg or more per day.]* |
|
*
For residual solvents with PDEs of more than 50mg per day,see the discussion in the section Class 3under Limits of Residual Solvents.
|
PROCEDURES FOR ESTABLISHING EXPOSURE LIMITS
The procedure used to establish permitted daily exposures for residual solvents is presented in Appendix 3.
OPTIONS FOR DETERMINING LEVELS OF CLASS2RESIDUAL SOLVENTS
Two options are available to determine levels of Class 2residual solvents.
Option 1
The concentration limits in ppm stated in Table 2are used.They were calculated using equation (1)below by assuming a product weight of 10g administered daily.
Here,PDEis given in terms of mg per day,and dose is given in g per day.
These limits are considered acceptable for all drug substances,excipients,and drug products.Therefore,this option may be applied if the daily dose is not known or fixed.If all drug substances and excipients in a formulation meet the limits given in Option 1,these components may be used in any proportion.No further calculation is necessary provided the daily dose does not exceed 10g.Products that are administered in doses greater than 10g per day are to be considered under Option 2.
Option 2
It is not necessary for each component of the drug product to comply with the limits given in Option 1.The PDEin terms of mg per day as stated in Table 2can be used with the known maximum daily dose and equation (1)above to determine the concentration of residual solvent allowed in a drug product.Such limits are considered acceptable provided that it has been demonstrated that the residual solvent has been reduced to the practical minimum.The limits should be realistic in relation to analytical precision,manufacturing capability,and reasonable variation in the manufacturing process.The limits should also reflect contemporary manufacturing standards.
Option 2may be applied by adding the amounts of a residual solvent present in each of the components of the drug product.The sum of the amounts of solvent per day should be less than that given by the PDE.
Consider an example of the application of Option 1and Option 2to acetonitrile concentration in a drug product.The permitted daily exposure to acetonitrile is 4.1mg per day;thus,the Option 1limit is 410ppm.The maximum administered daily weight of a drug product is 5.0g,and the drug product contains two excipients.The composition of the drug product and the calculated maximum content of residual acetonitrile are given in the following table.
| Component | Amount in Formulation (g) |
Acetonitrile Content (ppm) |
Daily Exposure (mg) |
| Drug substance |
0.3 | 800 | 0.24 |
| Excipient 1 | 0.9 | 400 | 0.36 |
| Excipient 2 | 3.8 | 800 | 3.04 |
| Drug product | 5.0 | 728 | 3.64 |
Excipient l meets the Option 1limit,but the drug substance,excipient 2,and drug product do not meet the Option 1limit.Nevertheless,the drug product meets the Option 2limit of 4.1mg per day and thus conforms to the acceptance criteria in this General Chapter.
Consider another example using acetonitrile as the residual solvent.The maximum administered daily weight of a drug product is 5.0g,and the drug product contains two excipients.The composition of the drug product and the calculated maximum content of residual acetonitrile are given in the following table.
| Component | Amount in Formulation (g) |
Acetonitrile Content (ppm) |
Daily Exposure (mg) |
| Drug substance |
0.3 | 800 | 0.24 |
| Excipient 1 | 0.9 | 2000 | 1.80 |
| Excipient 2 | 3.8 | 800 | 3.04 |
| Drug product | 5.0 | 1016 | 5.08 |
In this example,the drug product meets neither the Option 1nor the Option 2limit.The manufacturer could test the drug product to determine if the formulation process reduced the level of acetonitrile.If the level of acetonitrile was not reduced to the allowed limit during formulation,the product fails the requirements of the test.
LIMITS OF RESIDUAL SOLVENTS
Ethylene Oxide
[NOTE—The test for ethylene oxide is conducted only where specified in the individual monograph.]The standard solution parameters and the procedure for determination are described in the individual monograph.Unless otherwise specified in the individual monograph,the limit is 10µg per g.
Class 1
Class 1residual solvents (Table 1)should not be employed in the manufacture of drug substances,excipients,and drug products because of the unacceptable toxicities or deleterious environmental effects of these residual solvents.However,if Class 1residual solvents are used,their levels should be restricted as shown in Table 1,unless otherwise stated in the individual monograph.The solvent 1,1,1-trichloroethane is included in Table 1because it is an environmental hazard.The stated limit of 1500ppm is based on safety data.
When Class 1residual solvents are used in the manufacture of a drug substance,excipient,or drug product,the methodology described in the Identification,Control,and Quantification of Residual Solventssection of this General Chapter is to be applied wherever possible.Otherwise an appropriate validated procedure is to be employed.Such procedure shall be submitted to the USPfor inclusion in the relevant individual monograph.
Table 1.Class 1Residual Solvents
| Solvent | Concentration Limit (ppm) |
Concern |
| Benzene | 2 | Carcinogen |
| Carbon tetrachloride | 4 | Toxic and environ mental hazard |
| 1,2-Dichloroethane | 5 | Toxic |
| 1,1-Dichloroethene | 8 | Toxic |
| 1,1,1-Trichloroethane | 1500 | Environmental hazard |
Class 2
Class 2residual solvents (Table 2)should be limited in drug substances,excipients,and drug products because of the inherent toxicities of the residual solvents.PDEs are given to the nearest 0.1mg per day,and concentrations are given to the nearest 10ppm.The stated values do not reflect the necessary analytical precision of the determination procedure.Precision should be determined as part of the procedure validation.
If Class 2residual solvents are present at greater than their Option 1limits,they should be identified and quantified.The procedures described in the Identification,Control,and Quantification of Residual Solvents section of this General Chapter are to be applied wherever possible.Otherwise an appropriate validated procedure is to be employed.Such procedure shall be submitted to the USPfor inclusion in the relevant individual monograph.
NOTE—The following Class 2residual solvents are not readily detected by the headspace injection conditions described in the Identification,Control,and Quantification of Residual Solvents section of this General Chapter:formamide,2-ethoxyethanol,2-methoxyethanol,ethylene glycol,N-methylpyrrolidone,and sulfolane.Other appropriate validated procedures are to be employed for the control of these residual solvents.Such procedures shall be submitted to the USPfor inclusion in the relevant individual monograph.
Table 2.Class 2Residual Solvents
| Solvent | PDE (mg/day) |
Concentration limit (ppm) |
| Acetonitrile | 4.1 | 410 |
| Chlorobenzene | 3.6 | 360 |
| Chloroform | 0.6 | 60 |
| Cyclohexane | 38.8 | 3880 |
| 1,2-Dichloroethene | 18.7 | 1870 |
| 1,2-Dimethoxyethane | 1.0 | 100 |
| N,N-Dimethylacetamide | 10.9 | 1090 |
| N,N-Dimethylformamide | 8.8 | 880 |
| 1,4-Dioxane | 3.8 | 380 |
| 2-Ethoxyethanol | 1.6 | 160 |
| Ethylene glycol | 6.2 | 620 |
| Formamide | 2.2 | 220 |
| Hexane | 2.9 | 290 |
| Methanol | 30.0 | 3000 |
| 2-Methoxyethanol | 0.5 | 50 |
| Methylbutylketone | 0.5 | 50 |
| Methylcyclohexane | 11.8 | 1180 |
| Methylene chloride | 6.0 | 600 |
| N-Methylpyrrolidone | 5.3 | 530 |
| Nitromethane | 0.5 | 50 |
| Pyridine | 2.0 | 200 |
| Sulfolane | 1.6 | 160 |
| Tetrahydrofuran | 7.2 | 720 |
| Tetralin | 1.0 | 100 |
| Toluene | 8.9 | 890 |
| Trichloroethene | 0.8 | 80 |
| Xylene* | 21.7 | 2170 |
|
*
Usually 60%m-xylene,14%p-xylene,9%o-xylene with 17%ethyl benzene
|
||
Class 3
Class 3residual solvents (Table 3)may be regarded as less toxic and of lower risk to human health than Class 1and Class 2residual solvents.Class 3includes no solvent known as a human health hazard at levels normally accepted in pharmaceuticals.However,there are no long-term toxicity or carcinogenicity studies for many of the residual solvents in Class 3.Available data indicate that they are less toxic in acute or short-term studies and negative in genotoxicity studies.
Unless otherwise stated in the individual monograph,Class 3residual solvents are limited to not more than 50mg per day (corresponding to 5000ppm or 0.5%under Option 1).If a Class 3solvent limit in an individual monograph is greater than 50mg per day,that residual solvent should be identified and quantified.The procedures described in the Identification,Control,and Quantification of Residual Solvents section of this General Chapter are to be applied wherever possible.Otherwise an appropriate validated procedure is to be employed.Such procedure shall be submitted to the USPfor inclusion in the relevant individual monograph.
Table 3.Class 3Residual Solvents
(limited by GMPor other quality-based requirements in drug substances,excipients,and drug products)
(limited by GMPor other quality-based requirements in drug substances,excipients,and drug products)
| Acetic acid | Heptane |
| Acetone | Isobutyl acetate |
| Anisole | Isopropyl acetate |
| 1-Butanol | Methyl acetate |
| 2-Butanol | 3-Methyl-1-butanol |
| Butyl acetate | Methylethylketone |
| tert-Butylmethyl ether | Methylisobutylketone |
| Cumene | 2-Methyl-l-propanol |
| Dimethyl sulfoxide | Pentane |
| Ethanol | 1-Pentanol |
| Ethyl acetate | 1-Propanol |
| Ethyl ether | 2-Propanol |
| Ethyl formate | Propyl acetate |
| Formic acid |
Other Residual Solvents
The residual solvents listed in Table 4may also be of interest to manufacturers of drug substances,excipients,or drug products.However,no adequate toxicological data on which to base a PDEwas found.Specifications for these residual solvents will be provided in the respective individual monograph.
Table 4.Other Residual Solvents
(for which no adequate toxicological data was found)
(for which no adequate toxicological data was found)
| 1,1-Diethoxypropane | Methyl isopropyl ketone |
| 1,1-Dimethoxymethane | Methyltetrahydrofuran |
| 2,2-Dimethoxypropane | Solvent Hexane |
| Isooctane | Trichloroacetic acid |
| Isopropyl ether | Trifluoroacetic acid |
IDENTIFICATION,CONTROL,AND QUANTIFICATION OF RESIDUAL SOLVENTS
NOTE—The organic-free water specified in the following procedures produces no significantly interfering peaks when chromatographed.
Class 1and Class 2Residual Solvents
WATER-SOLUBLE ARTICLES
Procedure A—
Class 1Standard Stock Solution—
Transfer 1.0mLof USP Class 1Residual Solvents Mixture RSto a 100-mLvolumetric flask,add 9mLof dimethyl sulfoxide,dilute with water to volume,and mix.Transfer 1.0mLof this solution to a 100-mLvolumetric flask,dilute with water to volume,and mix.Transfer 1.0mLof this solution to a 10-mLvolumetric flask,dilute with water to volume,and mix.
Class 1Standard Solution—
Transfer 1.0mLof Class 1Standard Stock Solutionto an appropriate headspace vial,add 5.0mLof water,apply stopper,cap,and mix.
Class 2Standard Stock Solution—
Transfer 1.0mLof USP Class 2Residual Solvents Mixture RSto a 100-mLvolumetric flask,dilute with water to volume,and mix.
Class 2Standard Solution—
Transfer 1.0mLof Class 2Standard Stock Solutionto an appropriate headspace vial,add 5.0mLof water,apply stopper,cap,and mix.
Test Stock Solution—
Transfer about 250mg of the article under test,accurately weighed,to a 25-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.
Test Solution—
Transfer 5.0mLof Test Stock Solutionto an appropriate headspace vial,add 1.0mLof water,apply stopper,cap,and mix.
Class 1System Suitability Solution—
Transfer 1.0mLof Class 1Standard Stock Solutionto an appropriate headspace vial,add 5.0mLof Test Stock Solution,apply stopper,cap,and mix.
Chromatographic System (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector,a 0.32-mm ×30-m fused-silica column coated with a 1.8-µm layer of phase G43or a 0.53-mm ×30-m wide-bore column coated with a 3.0-µm layer of phase G43.The carrier gas is nitrogen or helium with a linear velocity of about 35cm per second,and a split ratio of 1:5.The column temperature is maintained at 40 for 20minutes,then raised at a rate of 10
for 20minutes,then raised at a rate of 10 per minute to 240
per minute to 240 ,and maintained at 240
,and maintained at 240 for 20minutes.The injection port and detector temperatures are maintained at 140
for 20minutes.The injection port and detector temperatures are maintained at 140 and 250
and 250 ,respectively.Chromatograph the Class 1Standard Solution,Class 1System Suitability Solution,and Class 2Standard Solution,and record the peak responses as directed for Procedure:the signal-to-noise ratio of 1,1,1-trichloroethane in the Class 1Standard Solutionis not less than 5;the signal-to-noise ratio of each peak in the Class 1System Suitability Solutionis not less than 3;and the resolution,R,between acetonitrile and methylene chloride in the Class 2Standard Solutionis not less than 1.0.
,respectively.Chromatograph the Class 1Standard Solution,Class 1System Suitability Solution,and Class 2Standard Solution,and record the peak responses as directed for Procedure:the signal-to-noise ratio of 1,1,1-trichloroethane in the Class 1Standard Solutionis not less than 5;the signal-to-noise ratio of each peak in the Class 1System Suitability Solutionis not less than 3;and the resolution,R,between acetonitrile and methylene chloride in the Class 2Standard Solutionis not less than 1.0.
Procedure—
Separately inject (following one of the headspace operating parameter sets described in the table below)equal volumes of headspace (about 1.0mL)of the Class 1Standard Solution,Class 2Standard Solution,and the Test Solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.If a peak response of any peak in the Test Solutionis greater than or equal to a corresponding peak in either the Class 1Standard Solutionor the Class 2Standard Solution,proceed to Procedure Bto verify the identity of the peak;otherwise the article meets the requirements of this test.
Table 5.Headspace Operating Parameters
| Headspace Operating Parameter Sets |
|||
| 1 | 2 | 3 | |
| Equilibration temperature ( |
80 | 105 | 80 |
| Equilibration time (min.) | 60 | 45 | 45 |
| Transfer-line temperature ( |
85 | 110 | 105 |
| Carrier gas:nitrogen or helium at an appropriate pressure | |||
| Pressurization time (s) | 30 | 30 | 30 |
| Injection volume (mL) | 1 | 1 | 1 |
Procedure B—
Class 1Standard Stock Solution,Class 1Standard Solution,Class 2Standard Stock Solution,Class 2Standard Solution,Test Stock Solution,Test Solution,andClass 1System Suitability Solution—
Prepare as directed for Procedure A.
Chromatographic System (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector,a 0.32-mm ×30-m fused-silica column coated with a 0.25-µm layer of phase G16,or a 0.53-mm ×30-m wide-bore column coated with a 0.25-µm layer of phase G16.The carrier gas is nitrogen or helium with a linear velocity of about 35cm per second and a split ratio of 1:5.The column temperature is maintained at 50 for 20minutes,then raised at a rate of 6
for 20minutes,then raised at a rate of 6 per minute to 165
per minute to 165 ,and maintained at 165
,and maintained at 165 for 20minutes.The injection port and detector temperatures are maintained at 140
for 20minutes.The injection port and detector temperatures are maintained at 140 and 250
and 250 ,respectively.Chromatograph the Class 1Standard Solution,the Class 1System Suitability Solution,and the Class 2Standard Solution,and record the peak responses as directed for Procedure:the signal-to-noise ratio of benzene in the Class 1Standard Solutionis not less than 5;the signal-to-noise ratio of each peak in the Class 1System Suitability Solutionis not less than 3;the resolution,R,between acetonitrile and trichloroethylene in the Class 2Standard Solutionis not less than 1.0.
,respectively.Chromatograph the Class 1Standard Solution,the Class 1System Suitability Solution,and the Class 2Standard Solution,and record the peak responses as directed for Procedure:the signal-to-noise ratio of benzene in the Class 1Standard Solutionis not less than 5;the signal-to-noise ratio of each peak in the Class 1System Suitability Solutionis not less than 3;the resolution,R,between acetonitrile and trichloroethylene in the Class 2Standard Solutionis not less than 1.0.
Procedure—
Separately inject (following one of the headspace operating parameter sets described in Table 5)equal volumes of headspace (about 1.0mL)of the Class 1Sandard Solution,the Class 2Standard Solution,and the Test Solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.If the peak response(s)in the Test Solutionof the peak(s)identified in Procedure Ais/are greater than or equal to a corresponding peak(s)in either the Class 1Standard Solutionor the Class 2Standard Solution,proceed to Procedure Cto quantify the peak;otherwise the article meets the requirements of this test.
Procedure C—
Class 1Standard Solution,Class 2Standard Solution,Test Stock Solution,Test Solution,andClass 1System Suitability Solution—
Prepare as directed for Procedure A.
Standard Solution—
Transfer an accurately measured volume of the USP Reference Standard for each peak identified and verified by Procedures Aand Bto a suitable container,and dilute quantitatively,and stepwise if necessary,with water to obtain a solution having a final concentration of 1/100of the value stated in Table 1or 2(under Concentration limit).Transfer 5.0mLof this solution to an appropriate headspace vial,add 1.0mLof water,apply stopper,cap,and mix.
Chromatographic System (see Chromatography á621ñ)—
[NOTE—If the results of the chromatography from Procedure Aare found to be inferior to those found with Procedure B,the Chromatographic Systemfrom Procedure Bmay be substituted.]The gas chromatograph is equipped with a flame-ionization detector,a 0.32-mm ×30-m fused-silica column coated with a 1.8-µm layer of phase G43or a 0.53-mm ×30-m wide-bore column coated with a 3.0-µm layer of phase G43.The carrier gas is nitrogen or helium with a linear velocity of about 35cm per second,and a split ratio of 1:5.The column temperature is maintained at 40 for 20minutes,then raised at a rate of 10
for 20minutes,then raised at a rate of 10 per minute to 240
per minute to 240 ,and maintained at 240
,and maintained at 240 for 20minutes.The injection port and detector temperatures are maintained at 140
for 20minutes.The injection port and detector temperatures are maintained at 140 and 250
and 250 ,respectively.Chromatograph the Class 1Standard Solution,the Class 1System Suitability Solution,and the Class 2Standard Solution,and record the peak responses as directed for Procedure:the signal-to-noise ratio of 1,1,1-trichloroethane in the Class 1Standard Solutionis not less than 5;the signal-to-noise ratio of each peak in the Class 1System Suitability Solutionis not less than 3;and the resolution,R,between acetonitrile and methylene chloride in the Class 2Standard Solutionis not less than 1.0.
,respectively.Chromatograph the Class 1Standard Solution,the Class 1System Suitability Solution,and the Class 2Standard Solution,and record the peak responses as directed for Procedure:the signal-to-noise ratio of 1,1,1-trichloroethane in the Class 1Standard Solutionis not less than 5;the signal-to-noise ratio of each peak in the Class 1System Suitability Solutionis not less than 3;and the resolution,R,between acetonitrile and methylene chloride in the Class 2Standard Solutionis not less than 1.0.
Procedure—
Separately inject (following one of the headspace operating parameters described in Table 5)equal volumes of headspace (about 1.0mL)of the Standard Solutionand Test Solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the amount,in ppm,of each residual solvent found in the article under test by the formula:
4(C/W)(rU/rS),
in which Cis the concentration,in ppm,of the appropriate USP Reference Standard in the Standard Solution;Wis the weight,in g,of the article under test taken to prepare the Test Stock Solution;and rUand rSare the peak responses of each residual solvent obtained from the Test Solution and the Standard Solution,respectively.
WATER-INSOLUBLE ARTICLES
Procedure A—
Class 1Standard Stock Solution,Class 1Standard Solution,Class 1System Suitability Solution,Class 2Standard Stock Solution,Class 2Standard Solution,andChromatographic System—
Proceed as directed for Procedure Aunder Water-Soluble Articles.
Test Stock Solution—
Transfer about 250mg of the article under test,accurately weighed,to a 25-mLvolumetric flask,dissolve in and dilute with dimethylformamide to volume,and mix.
Test Solution 1—
Transfer 5.0mLof Test Stock Solutionto an appropriate headspace vial,add 1.0mLof dimethylformamide,apply stopper,cap,and mix.
Test Solution 2—
Transfer about 250mg of the article under test,accurately weighed,to a 25-mLvolumetric flask,dissolve in and dilute with 1,3-dimethyl-2-imidazolidinone to volume,and mix.Transfer 5.0mLof this solution to an appropriate headspace vial,add 1.0mLof 1,3-dimethyl-2-imidazolidinone,apply stopper,cap,and mix.
Procedure—
Separately inject (following one of the headspace operating parameters described in Table 5)equal volumes of headspace (about 1.0mL)of the Class 1Standard Solution,the Class 2Standard Solution,Test Solution 1,and Test solution 2into the chromatograph,record the chromatograms,and measure the responses for the major peaks.If a peak response of any peak in Test solution 1is greater than or equal to a corresponding peak in either the Class 1Standard Solutionor the Class 2Standard Solution,proceed to Procedure Bto verify the identity of the peak;otherwise the article meets the requirements of this test.If the peak response for dimethylformamide or N,N-dimethylacetamide in Test Solution 2is greater than or equal to the corresponding peak in the Class 2Standard Solution,proceed to Procedure Bto verify the identity of the peak;otherwise the article meets the requirements of this test.
Procedure B—
Class 1Standard Stock Solution,Class 1Standard Solution,Class 2Standard Stock Solution,Class 2Standard Solution,and Class 1System Suitability Solution—
Prepare as directed for Procedure Aunder Water-Soluble Articles.
Test Stock Solution,Test Solution 1,and Test Solution 2—
Proceed as directed for Procedure A.
Chromatographic System—
Proceed as directed for Procedure Bunder Water-Soluble Articles.
Procedure—
Separately inject (following one of the headspace operating parameters described in Table 5)equal volumes of headspace (about 1.0mL)of the Class 1Standard Solution,Class 2Standard Solution,Test Solution 1,and/or Test Solution 2into the chromatograph,record the chromatograms,and measure the responses for the major peaks.If the peak response(s)in Test Solution 1of the peak(s)identified in Procedure Ais/are greater than or equal to a corresponding peak(s)in either the Class 1Standard Solution or the Class 2Standard Solution,proceed to Procedure Cto quantify the peak;otherwise the article meets the requirements of this test.If the peak response for dimethylformamide or N,N-dimethylacetamide in Test Solution 2is greater than or equal to the corresponding peak in the Class 2Standard Solution,proceed to Procedure Cto quantify the peak;otherwise the article meets the requirements of this test.
Procedure C—
Class 1Standard Solution,Class 1System Suitability Solution,andClass 2Standard Solution—
Proceed as directed for Procedure Aunder Water-Soluble Articles.
Test Stock Solution,Test Solution 1,andTest Solution 2—
Proceed as directed for Procedure A.
Standard Solution,andChromatographic System—
Proceed as directed for Procedure Cunder Water-Soluble Articles.
Procedure—
Separately inject (following one of the headspace operating parameters described in Table 5)equal volumes of headspace (about 1.0mL)of theStandard Solution,Test Solution 1,and/or Test Solution 2into the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the amount,in ppm,of each residual solvent found in the article under test by the formula:
4(C/W)(rU/rS),
in which Cis the concentration,in ppm,of the appropriate USP Reference Standard in the Standard Solution;Wis the weight,in g,of the article under test taken to prepare the Test Stock Solution;and rUand rSare the peak responses of each residual solvent obtained from Test Solution 1or Test Solution 2and the Standard Solution,respectively.
Class 3Residual Solvents
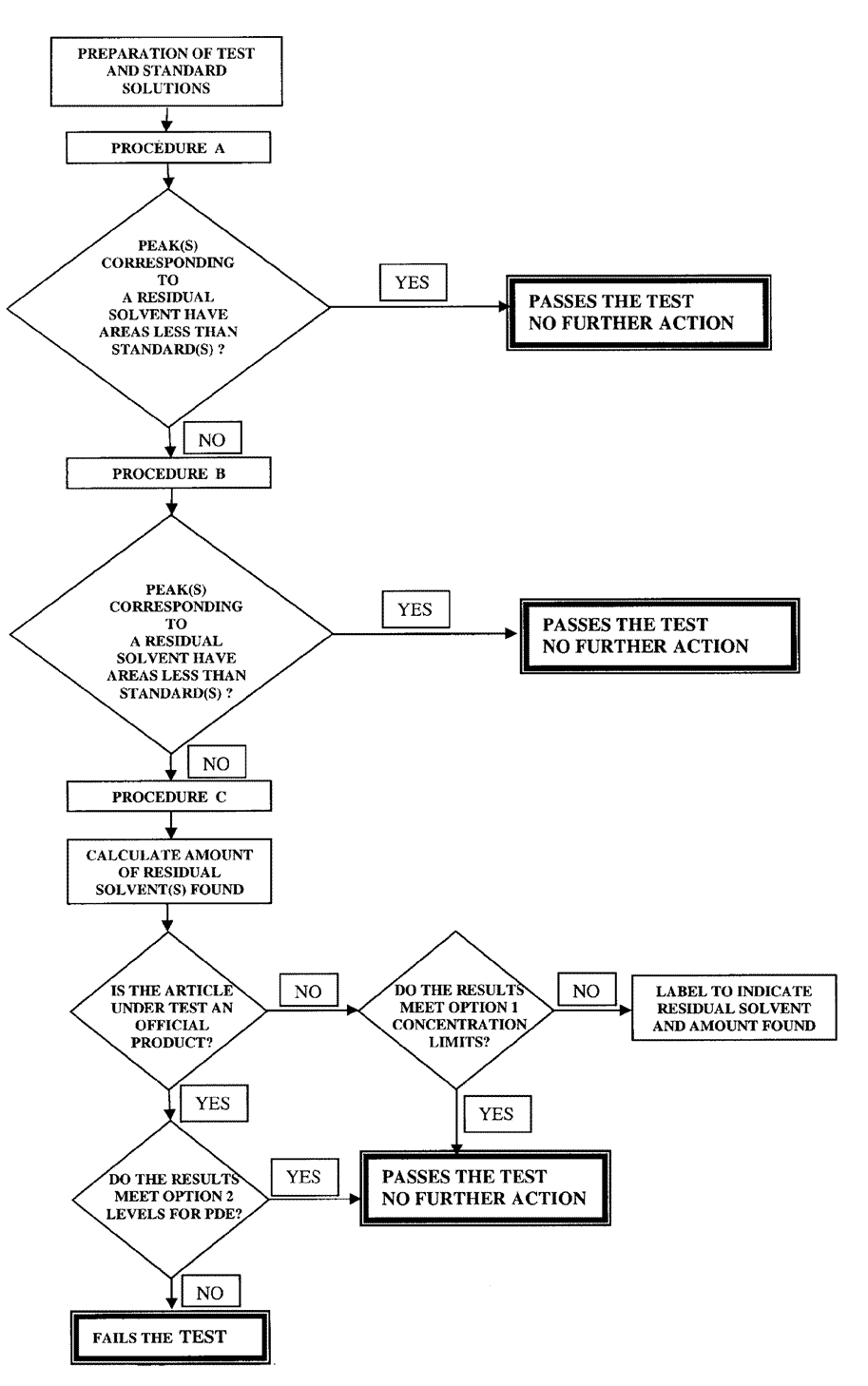
If only Class 3solvents are present,the level of residual solvents is to be determined as directed under Loss on Drying á731ñ.If the loss on drying value is greater than 0.5%,a water determination should be performed on the test sample as directed under Water Determination á921ñ.Determine the water by Method Ia,unless otherwise specified in the individual monograph.If a Class 3solvent limit in an individual monograph is greater than 50mg per day (corresponding to 5000ppm or 0.5%under Option 1),that residual solvent should be identified and quantified,and the procedures as described above are to be applied wherever possible.Otherwise an appropriate validated procedure is to be employed.Such procedure shall be submitted to the USPfor inclusion in the relevant individual monograph.Aflow diagram for the application of residual solvent limit tests is shown in Figure 1.
OTHER ANALYTICAL PROCEDURES
The following procedures,with any necessary variations,are used where specified in the individual monographs.
Method I
Agas chromatograph capable of temperature programming and equipped with a wide-bore,wall-coated open tubular column and a flame-ionization detector is used in the following procedure.
Standard Solution—
Prepare a solution,in organic-free water,or the solvent specified in the monograph,containing in each mL,12.0µg of methylene chloride,7.6µg of 1,4-dioxane,1.6µg of trichloroethylene,and 1.2µg of chloroform.[NOTE—Prepare fresh daily.]
Test Solution—
Dissolve in organic-free water,or the solvent specified in the monograph,an accurately weighed portion of the material to be tested to obtain a final solution having a known concentration of about 20mg of the test material per mL.
Chromatographic System (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector,a 0.53-mm ×30-m fused silica analytical column coated with a 5-µm chemically cross-linked G27stationary phase and a 0.53-mm ×5-m silica guard column deactivated with phenylmethyl siloxane.The carrier gas is helium with a linear velocity of about 35cm per second.[NOTE—When a makeup gas is used,nitrogen is recommended.]The injection port temperature and the detector temperature are maintained at 70 and 260
and 260 ,respectively.The column temperature is programmed as follows.Initially,the column temperature is maintained at 35
,respectively.The column temperature is programmed as follows.Initially,the column temperature is maintained at 35 for 5minutes,then increased at a rate of 8
for 5minutes,then increased at a rate of 8 per minute to 175
per minute to 175 ,followed by an increase at a rate of 35
,followed by an increase at a rate of 35 per minute to 260
per minute to 260 ,and maintained at 260
,and maintained at 260 for at least 16minutes.
for at least 16minutes.
Inject the Standard Solution,and record the peak responses as directed for Procedure:a suitable system is one that yields chromatograms in which all of the components in the Standard Solutionare resolved;the resolution,R,between any two components is not less than 1.0;and the relative standard deviation of the individual peak responses from replicate injections is not more than 15%.
Procedure—
Separately inject equal volumes (about 1µL)of the Standard Solutionand the Test Solutioninto the chromatograph,record the chromatograms,and measure the peak responses.
Identify,on the basis of retention time,any peaks present in the chromatogram of the Test Solution.The identity and peak response in the chromatogram may be established as being from any of the organic volatile impurities listed in the table shown below or from some other volatile impurity eluting with a comparable retention time as determined by mass spectrometric relative abundance procedures or by the use of a second validated column containing a different stationary phase.
Unless otherwise specified in the individual monograph,the amount of each organic volatile impurity present in the material does not exceed the limit given in the table shown below.
| Organic Volatile Impurity | Limit (µg per g) |
| Chloroform | 60 |
| 1,4-Dioxane | 380 |
| Methylene Chloride | 600 |
| Trichloroethylene | 80 |
Method IV
Standard Solution—
Prepare as directed for Standard Solutionin Method I.Pipet 5mLof the solution into a vial fitted with a septum and crimp cap,containing 1g of anhydrous sodium sulfate,and seal.Heat the sealed vial at 80 for 60minutes.
for 60minutes.
Test Solution—
Transfer 100mg,accurately weighed,of the material under test to a vial,add 5.0mLof water,or the solvent specified in the monograph,and 1g of anhydrous sodium sulfate,and seal with a septum and crimp cap.Heat the sealed vial at 80 for 60minutes,or as specified in the individual monograph.
for 60minutes,or as specified in the individual monograph.
Chromatographic System and Procedure—
[NOTE—The use of headspace apparatuses that automatically transfer a measured amount of headspace is allowed.Also,the use of a guard column in this headspace procedure is not necessary.]Proceed as directed for Method V,except to inject,using a heated gas-tight syringe,1mLof the headspace.
Method V
Standard Solution and Test Solution—
Prepare as directed for Method I.
Chromatographic System (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector,a 0.53-mm ×30-m fused silica analytical column coated with a 3.0-µm G43stationary phase,and a 0.53-mm ×5-m silica guard column deactivated with phenylmethyl siloxane.The carrier gas is helium with a linear velocity of about 35cm per second.The injection port and detector temperatures are maintained at 140 and 260
and 260 ,respectively.The column temperature is programmed according to the following steps.It is maintained at 40
,respectively.The column temperature is programmed according to the following steps.It is maintained at 40 for 20minutes,then increased rapidly to 240
for 20minutes,then increased rapidly to 240 ,and maintained at 240
,and maintained at 240 for 20minutes.
for 20minutes.
Inject the Standard Solution,and record the peak responses as directed for Procedure:a suitable system is one that yields chromatograms in which all of the components in the Standard Solutionare resolved;the resolution,R,between any two components is not less than 3;and the relative standard deviation of the individual peak responses from replicate injections is not more than 15%.
Procedure—
Proceed as directed for Method I,the injection volume being about 1µL.
Method VI
Standard Solution and Test Solution—
Prepare as directed for Method I.
Chromatographic System (see Chromatography á621ñ)—
The gas chromatograph is equipped with a flame-ionization detector.The column and column temperature conditions,as chosen from the list below (see Table 6),are specified in the individual monograph.The carrier gas,linear velocity or flow rate,and detector and injection port temperatures are appropriate to the column dimensions and column temperatures chosen from the list below.
Inject the Standard Solution,and record the peak responses as directed for Procedure:a suitable system is one that yields the chromatograms in which all of the components in the Standard Solutionare resolved;the resolution,R,between any two components is not less than 1.0;and the relative standard deviation of the individual peak responses from replicate injections is not more than 15%.
Procedure—
Proceed as directed for Method I,the injection volume being about 1µL.
Table 6.Chromatographic Conditions for Method VI
| Chromatographic Conditions |
USP Column Designation |
Column Size | Column Temperature |
| A | S3 | 3-mm ×2-m | 190 |
| B | S2 | 3-mm ×2.1-m | 160 |
| C | G16 | 0.53-mm ×30-m | 40 |
| D | G39 | 3-mm ×2-m | 65 |
| E | G16 | 3-mm ×2-m | 70 |
| F | S4 | 2-mm ×2.5-m | Hold 120 Gradient 120 Hold 20min. |
| H | G14 | 2-mm ×2.5-m | Hold 45 Gradient 45 Hold 15min. |
| I | G27 | 0.53-mm ×30-m | Hold 35 35 175 Hold 16min. |
| J | G16 | 0.33-mm ×30-m | Hold 50 50 Hold 20min. |
GLOSSARY
Genotoxic carcinogens:Carcinogens that produce cancer by affecting genes or chromosomes.
Lowest-observed-effect level(LOEL):The lowest dose of a substance in a study or group of studies that produces biologically significant increases in frequency or severity of any effects in exposed humans or animals.
Modifying factor:Afactor determined by professional judgment of a toxicologist and applied to bioassay data so that the data can be safely related to humans.
Neurotoxicity:The ability of a substance to cause adverse effects on the nervous system.
No-observed-effect level(NOEL):The highest dose of a substance at which there are no biologically significant increases in frequency or severity of any effects in exposed humans or animals.
Permitted daily exposure(PDE):The maximum acceptable intake per day of a residual solvent in pharmaceutical products.
Reversible toxicity:The occurrence of harmful effects that are caused by a substance and that disappear after exposure to the substance ends.
Strongly suspected human carcinogen:Asubstance for which there is no epidemiological evidence of carcinogenesis but for which there are positive genotoxicity data and clear evidence of carcinogenesis in rodents.
Teratogenicity:The occurrence of structural malformations in a developing fetus when a substance is administered during pregnancy.
APPENDIX1.LIST OF RESIDUAL SOLVENTS INCLUDED IN THIS GENERAL CHAPTER
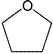
| Solvent | Other Names | Structure | Class |
| Acetic acid | Ethanoic acid | CH3COOH | Class 3 |
| Acetone | 2-Propanone Propan-2-one |
CH3COCH3 | Class 3 |
| Acetonitrile | CH3CN | Class 2 | |
| Anisole | Methoxybenzene | Class 3 | |
| Benzene | Benzol | Class 1 | |
| 1-Butanol | n-Butyl alcohol Butan-1-ol |
CH3(CH2)3OH | Class 3 |
| 2-Butanol | sec-Butyl alcohol Butan-2-ol |
CH3CH2CH(OH)CH3 | Class 3 |
| Butyl acetate | Acetic acid butyl ester | CH3COO(CH2)3CH3 | Class 3 |
| tert-Butylmethyl ether | 2-Methoxy-2-methylpropane | (CH3)3COCH3 | Class 3 |
| Carbon tetrachloride | Tetrachloromethane | CCl4 | Class 1 |
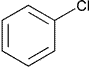
| Chlorobenzene | Class 2 | ||
| Chloroform | Trichloromethane | CHCl3 | Class 2 |
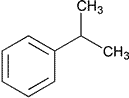
| Cumene | Isopropylbenzene (1-Methylethyl)benzene |
Class 3 | |
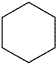
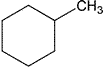
| Cyclohexane | Hexamethylene | Class 2 | |
| 1,2-Dichloroethane | sym-Dichloroethane Ethylene dichloride Ethylene chloride |
CH2ClCH2Cl | Class 1 |
| 1,1-Dichloroethene | 1,1-Dichloroethylene Vinylidene chloride |
H2C=CCl2 | Class 1 |
| 1,2-Dichloroethene | 1,2-Dichloroethylene Acetylene dichloride |
ClHC=CHCl | Class 2 |
| 1,2-Dimethoxyethane | Ethyleneglycol dimethyl ether Monoglyme Dimethyl cellosolve |
H3COCH2CH2OCH3 | Class 2 |
| N,N-Dimethylacetamide | DMA | CH3CON(CH3)2 | Class 2 |
| N,N-Dimethylformamide | DMF | HCON(CH3)2 | Class 2 |
| Dimethyl sulfoxide | Methylsulfinylmethane Methyl sulfoxide DMSO |
(CH3)2SO | Class 3 |
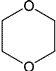
| 1,4-Dioxane | p-Dioxane [1,4]Dioxane |
Class 2 | |
| Ethanol | Ethyl alcohol | CH3CH2OH | Class 3 |
| 2-Ethoxyethanol | Cellosolve | CH3CH2OCH2CH2OH | Class 2 |
| Ethyl acetate | Acetic acid ethyl ester | CH3COOCH2CH3 | Class 3 |
| Ethylene glycol | 1,2-Dihydroxyethane 1,2-Ethanediol |
HOCH2CH2OH | Class 2 |
| Ethyl ether | Diethyl ether Ethoxyethane 1,1¢-Oxybisethane |
CH3CH2OCH2CH3 | Class 3 |
| Ethyl formate | Formic acid ethyl ester | HCOOCH2CH3 | Class 3 |
| Formamide | Methanamide | HCONH2 | Class 2 |
| Formic acid | HCOOH | Class 3 | |
| Heptane | n-Heptane | CH3(CH2)5CH3 | Class 3 |
| Hexane | n-Hexane | CH3(CH2)4CH3 | Class 2 |
| Isobutyl acetate | Acetic acid isobutyl ester | CH3COOCH2CH(CH3)2 | Class 3 |
| Isopropyl acetate | Acetic acid isopropyl ester | CH3COOCH(CH3)2 | Class 3 |
| Methanol | Methyl alcohol | CH3OH | Class 2 |
| 2-Methoxyethanol | Methyl cellosolve | CH3OCH2CH2OH | Class 2 |
| Methyl acetate | Acetic acid methyl ester | CH3COOCH3 | Class 3 |
| 3-Methyl-1-butanol | Isoamyl alcohol Isopentyl alcohol 3-Methylbutan-1-ol |
(CH3)2CHCH2CH2OH | Class 3 |
| Methylbutylketone | 2-Hexanone Hexan-2-one |
CH3(CH2)3COCH3 | Class 2 |
| Methylcyclohexane | Cyclohexylmethane | Class 2 | |
| Methylene chloride | Dichloromethane | CH2Cl2 | Class 2 |
| Methylethylketone | 2-Butanone MEK Butan-2-one |
CH3CH2COCH3 | Class 3 |
| Methyl isobutyl ketone | 4-Methylpentan-2-one 4-Methyl-2-pentanone MIBK |
CH3COCH2CH(CH3)2 | Class 3 |
| 2-Methyl-1-propanol | Isobutyl alcohol | (CH3)2CHCH2OH | Class 3 |
| 2-Methylpropan-1-ol | |||
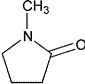
| N-Methylpyrrolidone | 1-Methylpyrrolidin-2-one 1-Methyl-2-pyrrolidinone |
Class 2 | |
| Nitromethane | CH3NO2 | Class 2 | |
| Pentane | n-Pentane | CH3(CH2)3CH3 | Class 3 |
| 1-Pentanol | Amyl alcohol Pentan-1-ol Pentyl alcohol |
CH3(CH2)3CH2OH | Class 3 |
| 1-Propanol | Propan-1-ol Propyl alcohol |
CH3CH2CH2OH | Class 3 |
| 2-Propanol | Propan-2-ol Isopropyl alcohol |
(CH3)2CHOH | Class 3 |
| Propyl acetate | Acetic acid propyl ester | CH3COOCH2CH2CH3 | Class 3 |
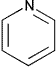
| Pyridine | Class 2 | ||
| Sulfolane | Tetrahydrothiophene 1,1-diox- ide |
Class 2 | |
| Tetrahydrofuran | Tetramethylene oxide | Class 2 | |
| Oxacyclopentane | |||
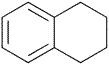
| Tetralin | 1,2,3,4-Tetrahydronaphthalene | Class 2 | |
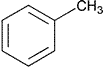
| Toluene | Methylbenzene | Class 2 | |
| 1,1,1-Trichloroethane | Methylchloroform | CH3CCl3 | Class 1 |
| Trichloroethene | 1,1,2-Trichloroethene | HClC=CCl2 | Class 2 |
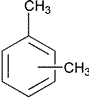
| Xylene* | Dimethybenzene Xylol |
Class 2 | |
|
*
Usually 60%m-xylene,14%p-xylene,9%o-xylene with 17%ethyl benzene.
|
|||
APPENDIX2.ADDITIONAL BACKGROUND
A2.1.Environmental Regulation of Organic Volatile Solvents
Several of the residual solvents frequently used in the production of pharmaceuticals are listed as toxic chemicals in Environmental Health Criteria (EHC)monographs and in the Integrated Risk Information System (IRIS).The objectives of such groups as the International Programme on Chemical Safety (IPCS),the United States Environmental Protection Agency (EPA),and the United States Food and Drug Administration (FDA)include the determination of acceptable exposure levels.The goal is maintenance of environmental integrity and protection of human health against the possible deleterious effects of chemicals resulting from long-term environmental exposure.The procedures involved in the estimation of maximum safe exposure limits are usually based on long-term studies.When long-term study data are unavailable,shorter term study data can be used with modification of the approach,such as use of larger safety factors.The approach described therein relates primarily to long-term or lifetime exposure of the general population in the ambient environment (i.e.,ambient air,food,drinking water,and other media).
A2.2.Residual Solvents in Pharmaceuticals
Exposure limits in this General Chapter are established by referring to methodologies and toxicity data described in EHCand IRISmonographs.However,the following specific assumptions about residual solvents to be used in the synthesis and formulation of pharmaceutical products should be taken into account in establishing exposure limits.
-
Patients (not the general population)use pharmaceuticals to treat their diseases or for prophylaxis to prevent infection or disease.
-
The assumption of lifetime patient exposure is not necessary for most pharmaceutical products but may be appropriate as a working hypothesis to reduce risk to human health.
-
Residual solvents are unavoidable components in pharmaceutical production and will often be a part of medicinal products.
-
Residual solvents should not exceed recommended levels except in exceptional circumstances.
-
Data from toxicological studies that are used to determine acceptable levels for residual solvents should have been generated using appropriate protocols such as those described,for example,by the Organization for Economic Cooperation and Development (OECD),EPA,and the FDA Red Book.
APPENDIX3.PROCEDURES FOR ESTABLISHING EXPOSURE LIMITS
The Gaylor-Kodell method of risk assessment (Gaylor,D.W.and Kodell,R.L.Linear Interpolation Algorithm for Low Dose Assessment of Toxic Substance.Journal of Environmental Pathology and Toxicology,4:305,1980)is appropriate for Class 1carcinogenic solvents.Only in cases where reliable carcinogenicity data are available should extrapolation by the use of mathematical models be applied to setting exposure limits.Exposure limits for Class 1residual solvents could be determined with the use of a large safety factor (i.e.,10,000to 100,000)with respect to the no-observed-effect level (NOEL).Detection and quantification of these residual solvents should be performed by state-of-the-art analytical techniques.
Acceptable exposure levels in this General Chapter for Class 2residual solvents were established by calculation of PDEvalues according to the procedures for setting exposure limits in pharmaceuticals (page 5748of PF15(6)[Nov.–Dec.1989]),and the method adopted by IPCSfor Assessing Human Health Risk of Chemicals (Environmental Health Criteria 170,WHO,1994).These procedures are similar to those used by the U.S.EPA(IRIS)and the U.S.FDA(Red Book)and others.The method is outlined here to give a better understanding of the origin of the PDEvalues.It is not necessary to perform these calculations in order to use the PDEvalues presented in Table 2of this document.
PDEis derived from the no-observed-effect level (NOEL),or the lowest-observed effect level (LOEL),in the most relevant animal study as follows:
The PDEis derived preferably from a NOEL.If no NOELis obtained,the LOELmay be used.Modifying factors proposed here,for relating the data to humans,are the same kind of “uncertainty factors”used in Environmental Health Criteria (Environmental Health Criteria 170,WHO,Geneva,1994)and “modifying factors”or “safety factors”in Pharmacopeial Forum.The assumption of 100percent systemic exposure is used in all calculations regardless of route of administration.
The modifying factors are as follows:
| F1= | Afactor to account for extrapolation between species | |
| F1= | 2for extrapolation from dogs to humans | |
| F1= | 2.5for extrapolation from rabbits to humans | |
| F1= | 3for extrapolation from monkeys to humans | |
| F1= | 5for extrapolation from rats to humans | |
| F1= | 10for extrapolation from other animals to humans | |
| F1= | 12for extrapolation from mice to humans | |
F1takes into account the comparative surface area to body weight ratios for the species concerned and for man.Surface area (S)is calculated as:
S=kM0.67,(2)
in whichM=body weight,and the constant khas been taken to be 10.The body weights used in the equation are those shown below in Table A3.-1.
| F2= | Afactor of 10to account for variability between individuals.Afactor of 10is generally given for all organic solvents,and 10is used consistently in this General Chapter. |
| F3= | Avariable factor to account for toxicity studies of short-term exposure. | |
| F3= | 1for studies that last at least one half-lifetime (1year for rodents or rabbits;7years for cats,dogs,and monkeys). | |
| F3= | 1for reproductive studies in which the whole period of organogenesis is covered. | |
| F3= | 2for a 6-month study in rodents,or a 3.5-year study in nonrodents. | |
| F3= | 5for a 3-month study in rodents,or a 2-year study in nonrodents. | |
| F3= | 10for studies of a shorter duration. | |
In all cases,the higher factor has been used for study durations between the time points (e.g.,a factor of 2for a 9-month rodent study).
| F4= | Afactor that may be applied in cases of severe toxicity,e.g.,nongenotoxic carcinogenicity,neurotoxicity,or teratogenicity.In studies of reproductive toxicity,the following factors are used: | |
| F4= | 1for fetal toxicity associated with maternal toxicity | |
| F4= | 5for fetal toxicity without maternal toxicity | |
| F4= | 5for a teratogenic effect with maternal toxicity | |
| F4= | 10for a teratogenic effect without maternal toxicity | |
| F5= | Avariable factor that may be applied if the no-effect level was not established. |
When only a LOELis available,a factor of up to 10can be used depending on the severity of the toxicity.The weight adjustment assumes an arbitrary adult human body weight for either sex of 50kilograms (kg).This relatively low weight provides an additional safety factor against the standard weights of 60kg or 70kg that are often used in this type of calculation.It is recognized that some adult patients weigh less than 50kg;these patients are considered to be accommodated by the built-in safety factors used to determine a PDE.If the solvent was present in a formulation specifically intended for pediatric use,an adjustment for a lower body weight would be appropriate.
As an example of the application of this equation,consider a toxicity study of acetonitrile in mice that is summarized in Pharmeuropa,Vol.9,No.1,Supplement,April 1997,page S24.The NOELis calculated to be 50.7mg kg–1day–l.The PDEfor acetonitrile in this study is calculated as follows:
In this example,
| F1= | 12to account for the extrapolation from mice to humans |
| F2= | 10to account for differences between individual humans |
| F3= | 5because the duration of the study was only 13weeks |
| F4= | 1because no severe toxicity was encountered |
| F5= | 1because the no-effect level was determined |
A3.-1.-Values Used in the Calculations in This Document
| Rat body weight | 425g |
| Pregnant rat body weight | 330g |
| Mouse body weight | 28g |
| Pregnant mouse body weight | 30g |
| Guinea-pig body weight | 500g |
| Rhesus monkey body weight | 2.5kg |
| Rabbit body weight (pregnant or not) | 4kg |
| Beagle dog body weight | 11.5kg |
| Rat respiratory volume | 290L/day |
| Mouse respiratory volume | 43L/day |
| Rabbit respiratory volume | 1440L/day |
| Guinea-pig respiratory volume | 430Lday |
| Human respiratory volume | 28,800L/day |
| Dog respiratory volume | 9000L/day |
| Monkey respiratory volume | 1150L/day |
| Mouse water consumption | 5mL/day |
| Rat water consumption | 30mL/day |
| Rat food consumption | 30g/day |
The equation for an ideal gas,PV=nRT,is used to convert concentrations of gases used in inhalation studies from units of ppm to units of mg/Lor mg/m3.Consider as an example the rat reproductive toxicity study by inhalation of carbon tetrachloride (molecular weight 153.84)summarized in Pharmeuropa,Vol.9,No.1,Supplement,April 1997,page S9.
The relationship 1000L=1m3is used to convert to mg/m3.