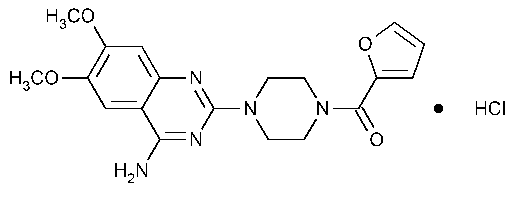
Prazosin Hydrochloride
Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)-,monohydrochloride.
1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furoyl)piperazine monohydrochloride [19237-84-4].
»Prazosin Hydrochloride contains not less than 97.0percent and not more than 103.0percent of C19H21N5O4·HCl,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Labeling—
Label it to indicate whether it is anhydrous or is the polyhydrate.
Identification—
A:
Infrared Absorption á197Kñ—Obtain the test specimen as follows.Dissolve about 20mg of it in 20mLof methanol,with the aid of gentle heat,and evaporate to dryness.Dry the residue in vacuum at 130 for 3hours.Proceed as directed with the residue so obtained and a similar preparation of USP Prazosin Hydrochloride RS.
for 3hours.Proceed as directed with the residue so obtained and a similar preparation of USP Prazosin Hydrochloride RS.
B:
Ultraviolet Absorption á197Uñ—
Solution:
7µg per mL.
Medium:
methanolic 0.01Nhydrochloric acid.
Absorptivities at 329nm and 246nm,calculated on the anhydrous basis,do not differ by more than 4.0%.
C:
Prepare a test solution of it in a mixture of chloroform,methanol,and diethylamine (10:10:1)containing 5mg per mL,and proceed as directed under Thin-layer Chromatographic Identification Test á201ñ,using a solvent system consisting of a mixture of ethyl acetate and diethylamine (19:1).
D:
It responds to the tests for Chloride á191ñ.
Water,Method Iá921ñ:
not more than 2.0%for the anhydrous form;between 8.0%and 15.0%for the polyhydrate form.
Residue on ignition á281ñ:
not more than 0.4%,determined on a 1-g portion,accurately weighed.
Heavy metals,Method IIá231ñ:
0.005%.
Iron—
Standard preparation—
Dissolve 100mg of iron wire,accurately weighed,in 10mLof hydrochloric acid,with boiling.Cool,transfer to a 1000-mLvolumetric flask,dilute with water to volume,and mix.Dilute quantitatively and stepwise with 0.2Nnitric acid to obtain a solution containing 4.0µg of iron per mL.
Test preparation—
Dissolve the residue obtained in the test for Residue on ignitionin 20mLof 2Nnitric acid.Slowly evaporate this solution to approximately 5mL,transfer to a 25-mLvolumetric flask,using 0.2Nnitric acid as a wash solvent,dilute with 0.2Nnitric acid to volume,and mix.
Procedure—
Concomitantly determine the absorbances of the Standard preparationand the Test preparationat the wavelength of maximum absorbance at about 248nm,with a suitable atomic absorption spectrophotometer (see Apparatusunder Spectrophotometry and Light-scattering á851ñ)equipped with an iron hollow-cathode lamp and an air–acetylene flame,using water as the blank:the absorbance of the Test preparationis not more than that of the Standard preparation(0.010%).
Nickel—
Standard preparation—
Dissolve 100mg of nickel,accurately weighed,in 10mLof nitric acid with the aid of boiling.Cool,transfer to a 1000-mLvolumetric flask,dilute with water to volume,and mix.Dilute quantitatively and stepwise with 0.2Nnitric acid to obtain a solution containing 4.0µg of nickel per mL.
Procedure—
Concomitantly determine the absorbances of the Standard preparationand the Test preparationat the wavelength of maximum absorbance at about 232nm,with a suitable atomic absorption spectrophotometer (see Apparatusunder Spectrophotometry and Light-scattering á851ñ)equipped with a nickel hollow-cathode lamp and an air–acetylene flame,using water as the blank:the absorbance of the Test preparationis not more than that of the Standard preparation(0.010%).
Ordinary impurities á466ñ—
Test solution:
a mixture of chloroform,methanol,and diethylamine (10:10:1).
Standard solution:
a mixture of chloroform,methanol,and diethylamine (10:10:1).
Eluant:
a mixture of ethyl acetate and diethylamine (19:1).
Visualization:
1.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
Mobile phase—
Mix 700mLof methanol,300mLof water,and 10mLof glacial acetic acid.Add diethylamine in sufficient quantity (about 0.2mL)such that the retention time of prazosin hydrochloride is between 6and 10minutes.Degas the solution.
Standard preparation—
Transfer about 100mg of USP Prazosin Hydrochloride RS,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with methanol to volume,and mix.Dilute this solution quantitatively with a mixture of methanol and water (7:3)to obtain a solution having a known concentration of about 30µg per mL.
Assay preparation—
Transfer about 100mg of Prazosin Hydrochloride,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with methanol to volume,and mix.Pipet 3mLof this solution into a 100-mLvolumetric flask,dilute with a mixture of methanol and water (7:3)to volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column that contains packing L3.The flow rate is adjusted to obtain a retention time of between 6and 10minutes for prazosin hydrochloride.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Inject equal volumes (about 5µL)of the Standard preparationand the Assay preparationinto the chromatograph,using a suitable microsyringe or sampling valve,and measure the peak responses at identical retention times.Calculate the quantity,in mg,of C19H21N5O4·HCl in the Prazosin Hydrochloride taken by the formula:
(C/0.3)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Prazosin Hydrochloride RS,calculated on the anhydrous basis,in the Standard preparation,and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 1606
Phone Number:1-301-816-8305