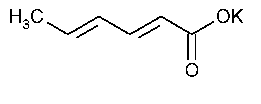
Potassium Sorbate
2,4-Hexadienoic acid,(E,E)-,potassium salt;2,4-Hexadienoic acid,potassium salt.
Potassium (E,E)-sorbate;Potassium sorbate [590-00-1;24634-61-5].
»Potassium Sorbate contains not less than 98.0percent and not more than 101.0percent of C6H7KO2,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers,protected from light,and avoid exposure to excessive heat.
Identification—
A:
Dissolve 1g in 10mLof water:the solution responds to the test for Potassium á191ñ.
B:
Dissolve 0.2g in 2mLof water,and add a few drops of bromine TS:the color is discharged.
Acidity or alkalinity—
Dissolve 1.1g in 20mLof water,and add phenolphthalein TS.If the solution is colorless,titrate with 0.10Nsodium hydroxide to a pink color that persists for 15seconds:not more than 1.1mLis required.If the solution is pink in color,titrate with 0.10Nhydrochloric acid:not more than 0.80mLis required to discharge the pink color.
Loss on drying á731ñ—
Dry it at 105 for 3hours:it loses not more than 1.0%of its weight.
for 3hours:it loses not more than 1.0%of its weight.
Heavy metals,Method IIá231ñ:
0.001%.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 300mg of Potassium Sorbate,accurately weighed,in 40mLof glacial acetic acid,warming,if necessary,to effect solution.Cool to room temperature,add 1drop of crystal violet TS,and titrate with 0.1Nperchloric acid VSto a blue-green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 15.02mg of C6H7KO2.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 3066
Phone Number:1-301-816-8379