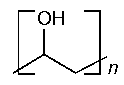
Polyvinyl Alcohol
»Polyvinyl Alcohol is a water-soluble synthetic resin,represented by the formula:
(C2H4O)n,
in which the average value of nlies between 500and 5000.It is prepared by 85percent to 89percent hydrolysis of polyvinyl acetate.The apparent viscosity,in centipoises,at 20 ,of a solution containing 4g of Polyvinyl Alcohol in each 100g is not less than 85.0percent and not more than 115.0percent of that stated on the label.
,of a solution containing 4g of Polyvinyl Alcohol in each 100g is not less than 85.0percent and not more than 115.0percent of that stated on the label.
Packaging and storage—
Preserve in well-closed containers.
Viscosity—
After determining the Loss on drying,weigh a quantity of undried Polyvinyl Alcohol,equivalent to 6.00g on the dried basis.Over a period of seconds,transfer the test specimen with continuous slow stirring to about 140mLof water contained in a suitable tared flask.When the specimen is well-wetted,increase the rate of stirring,avoiding mixing in excess air.Heat the mixture to 90 ,and maintain the temperature at 90
,and maintain the temperature at 90 for about 5minutes.Discontinue heating,and continue stirring for 1hour.Add water to make the mixture weigh 150g.Resume stirring to obtain a homogenous solution.Filter the solution through a tared 100-mesh screen into a 250-mLconical flask,cool to about 15
for about 5minutes.Discontinue heating,and continue stirring for 1hour.Add water to make the mixture weigh 150g.Resume stirring to obtain a homogenous solution.Filter the solution through a tared 100-mesh screen into a 250-mLconical flask,cool to about 15 ,mix,and proceed as directed under Viscosity á911ñ.
,mix,and proceed as directed under Viscosity á911ñ.
pHá791ñ:
between 5.0and 8.0,in a solution (1in 25).
Loss on drying á731ñ—
Dry it at 110 to constant weight:it loses not more than 5.0%of its weight.
to constant weight:it loses not more than 5.0%of its weight.
Residue on ignition á281ñ:
not more than 2.0%.
Water-insoluble substances—
Wash the tared 100-mesh screen used in the test for Viscositywith two 25-mLportions of water,and dry at 110 for 1hour:not more than 6.4mg of water-insoluble substances is found (0.1%).
for 1hour:not more than 6.4mg of water-insoluble substances is found (0.1%).
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Degree of hydrolysis—
Procedure—
Transfer about 1g of Polyvinyl Alcohol,previously dried at 110 to constant weight and accurately weighed,to a wide-mouth,250-mLconical flask fitted by means of a suitable glass joint to a reflux condenser.Add 35mLof dilute methanol (3in 5),and mix gently to assure complete wetting of the solid.Add 3drops of phenolphthalein TS,and add 0.2Nhydrochloric acid or 0.2Nsodium hydroxide,if necessary,to neutralize.Add 25.0mLof 0.2Nsodium hydroxide VS,and reflux gently on a hot plate for 1hour.Wash the condenser with 10mLof water,collecting the washings in the flask,cool,and titrate with 0.2Nhydrochloric acid VS.Concomitantly perform a blank determination in the same manner,using the same quantity of 0.2Nsodium hydroxide VS.
to constant weight and accurately weighed,to a wide-mouth,250-mLconical flask fitted by means of a suitable glass joint to a reflux condenser.Add 35mLof dilute methanol (3in 5),and mix gently to assure complete wetting of the solid.Add 3drops of phenolphthalein TS,and add 0.2Nhydrochloric acid or 0.2Nsodium hydroxide,if necessary,to neutralize.Add 25.0mLof 0.2Nsodium hydroxide VS,and reflux gently on a hot plate for 1hour.Wash the condenser with 10mLof water,collecting the washings in the flask,cool,and titrate with 0.2Nhydrochloric acid VS.Concomitantly perform a blank determination in the same manner,using the same quantity of 0.2Nsodium hydroxide VS.
Calculation of saponification value—
Calculate the saponification value by the formula:
[(B-A)N56.11]/W,
in which Band Aare the volumes,in mL,of 0.2Nhydrochloric acid VSconsumed in the titration of the blank and the test preparation,respectively,Nis the exact normality of the hydrochloric acid solution,Wis the weight,in g,of the portion of Polyvinyl Alcohol taken,and 56.11is the molecular weight of potassium hydroxide.
Calculation of degree of hydrolysis—
Calculate the degree of hydrolysis,expressed as percentage of hydrolysis of polyvinyl acetate,by the formula:
100-[7.84S/(100-0.075S)],
in which Sis the saponification value of the Polyvinyl Alcohol taken:between 85%and 89%is found.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 1579
Phone Number:1-301-816-8379