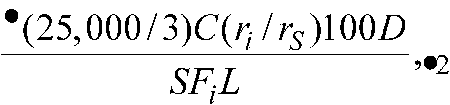Atovaquone Oral Suspension
»Atovaquone Oral Suspension contains not less than 90.0percent and not more than 110.0percent of the labeled amount of atovaquone (C22H19ClO3).
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:Ultraviolet Absorption á197Uñ—
Medium:
a mixture of methanol and water (1:1).
Solution—
Transfer 5.0mLof theAssay preparationand 5.0mLof theStandard preparation,prepared in theAssay,to separate 50-mLvolumetric flasks,dilute withMediumto volume,and mix.
B:
The retention time of the major peak in the chromatogram of theAssay preparation corresponds to that in the chromatogram of theStandard preparation,as obtained in the Assay.
Uniformity of dosage units á905ñ—
FOR ORAL SUSPENSION PACKAGED IN SINGLE-UNIT CONTAINERS:
meets the requirements.
Deliverable volume á698ñ—
FOR ORAL SUSPENSION PACKAGED IN MULTIPLE-UNIT CONTAINERS:
meets the requirements.
pHá791ñ:
between 3.5and 7.0.
Change to read:
Sedimentation—
Related compounds—
Using the chromatograms of theResolution solution,the Standard preparation,and the Assay preparation obtained in theAssay,calculate the percentage of atovaquone-related compounds,based on the labeled strength of atovaquone,by the formula:
in whichCis the concentration,in mg per mL,of USP Atovaquone RSin theStandard preparation;Dis the density of Oral Suspension,in g per mL(1.04g per mLat 20 to 25
to 25 );Sis the weight,in g,of Oral Suspension taken to prepare the Assay preparation;Lis the labeled amount,in mg per mL,of atovaquone in the Oral Suspension;Fiis the response factor of an individual atovaquone related compound relative to the response of atovaquone,specifically,1.08for any peak observed at a relative retention time of about 0.65,0.85for any peak observed at a retention time corresponding to that of atovaquone related compound A,as determined from the chromatogram of theResolution solution,and 1.0for any other related compound peak;riis the individual peak response of an atovaquone related compound,if any,in the chromatogram of theAssay preparation;andrSis the peak response of atovaquone in the chromatogram of theStandard preparation.Disregard any peak having a relative retention time of about 0.3,which is due to photodegradation during preparation of theAssay preparation.Not more than 0.5%of an atovaquone related compound with a relative retention time of about 0.65is found;not more than 1.0%of atovaquone related compound Ais found;not more than 0.3%of an atovaquone related compound with a relative retention time of about 0.88is found;not more than 0.2%of any other atovaquone related compound is found;and the sum of all related compounds is not more than 2.0%.
);Sis the weight,in g,of Oral Suspension taken to prepare the Assay preparation;Lis the labeled amount,in mg per mL,of atovaquone in the Oral Suspension;Fiis the response factor of an individual atovaquone related compound relative to the response of atovaquone,specifically,1.08for any peak observed at a relative retention time of about 0.65,0.85for any peak observed at a retention time corresponding to that of atovaquone related compound A,as determined from the chromatogram of theResolution solution,and 1.0for any other related compound peak;riis the individual peak response of an atovaquone related compound,if any,in the chromatogram of theAssay preparation;andrSis the peak response of atovaquone in the chromatogram of theStandard preparation.Disregard any peak having a relative retention time of about 0.3,which is due to photodegradation during preparation of theAssay preparation.Not more than 0.5%of an atovaquone related compound with a relative retention time of about 0.65is found;not more than 1.0%of atovaquone related compound Ais found;not more than 0.3%of an atovaquone related compound with a relative retention time of about 0.88is found;not more than 0.2%of any other atovaquone related compound is found;and the sum of all related compounds is not more than 2.0%.
Assay—
Mobile phase—
Prepare a mixture of acetonitrile,water,methanol,and phosphoric acid (480:360:160:5).Make adjustments if necessary (seeSystem Suitability under Chromatography á621ñ).
Resolution solution—
Prepare a solution in 0.1Mmethanolic sodium hydroxide containing about 0.09mg of USP Atovaquone RSand 0.01mg of USP Atovaquone Related Compound A RSper mL.Store in a low-actinic glass container.
Standard preparation—
Transfer about 30mg of USP Atovaquone RS,accurately weighed,to a low-actinic 10-mLvolumetric flask,and add 2mLof water and 6mLof 0.1Mmethanolic sodium hydroxide.Sonicate for about 5minutes or until the material has dissolved.Allow to cool,dilute with 0.1Mmethanolic sodium hydroxide to volume,and mix.Transfer 3.0mLof this solution to a low-actinic 100-mLvolumetric flask,dilute with a mixture of methanol and water (1:1),and mix.[NOTE—Minimize exposure of this solution to light.]
Assay preparation—
Transfer approximately 5.2g of the well-mixed Oral Suspension,accurately weighed,to a low-actinic 250-mLvolumetric flask.Add 50mLof water,swirl for about 5minutes,add 150mLof 0.1Mmethanolic sodium hydroxide,and sonicate for about 15minutes.Allow to cool,dilute with 0.1Mmethanolic sodium hydroxide to volume,and mix.Immediately filter a 20-mLportion,discarding the first 5mLof the filtrate.Transfer 3.0mLof the clear filtrate to a low-actinic 100-mLvolumetric flask,dilute with a mixture of methanol and water (1:1)to volume,and mix.[NOTE—Minimize exposure of this solution to light.]
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 220-nm detector and a 4.6-mm ×12.5-cm column that contains packing L1.The flow rate is about 3mLper minute.Chromatograph theResolution solution,and record the peak areas as directed forProcedure:the relative retention times are about 0.86for atovaquone related compound Aand 1.0for atovaquone.Chromatograph theStandard preparation,and record the peak areas as directed forProcedure:the tailing factor is not more than 1.5;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of theStandard preparation,the Resolution solution,and the Assay preparation into the chromatograph,record the chromatograms,and measure the areas for the major peaks.Calculate the quantity,in mg,of atovaquone (C22H19ClO3)in each mLof the Oral Suspension taken by the formula:
(25,000/3)(C/V)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Atovaquone RSin theStandard preparation;Vis the volume,in mL,of Oral Suspension taken to prepare theAssay preparation;andrUandrSare the atovaquone peak areas obtained from theAssay preparation and theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 197
Pharmacopeial Forum:Volume No.30(2)Page 449
Phone Number:1-301-816-8394