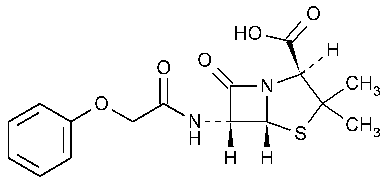
Penicillin V
4-Thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid,3,3-dimethyl-7-oxo-6-(phenoxyacetyl)amino-,2S-(2a,5a,6b)-.
(2S,5R,6R)-3,3-Dimethyl-7-oxo-6-(2-phenoxyacetamido)-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid [87-08-1].
»Penicillin Vhas a potency of not less than 1525and not more than 1780Penicillin V Units per mg.
Packaging and storage—
Preserve in tight containers.
Labeling—
Label it to indicate that it is to be used in the manufacture of nonparenteral drugs only.
Identification,Infrared Absorption á197Kñ—
Do not dry specimens.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 2.5and 4.0,in a suspension containing 30mg per mL.
Water,Method Iá921ñ:
not more than 2.0%.
Phenoxyacetic acid—
Diluent—
Use pH6.6phosphate buffer (see Buffer Solutionsin the section Reagents,Indicators,and Solutions).
Mobile phase—
Prepare a mixture of water,acetonitrile,and glacial acetic acid (65:35:1).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of phenoxyacetic acid quantitatively in Diluentto obtain a solution having a known concentration of about 0.1mg per mL.
Test solution—
Dissolve an accurately weighed quantity of Penicillin Vquantitatively in Diluentto obtain a solution containing 20.0mg per mL.[NOTE—Use this solution on the day prepared.]
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column containing 5-µm packing L1.The flow rate is about 1mLper minute.Chromatograph the Standard solution,and record the responses as directed for Procedure:the tailing factor is not more than 1.5,and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the phenoxyacetic acid peaks.Calculate the percentage of phenoxyacetic acid in the portion of Penicillin Vtaken by the formula:
5C(rU/rS),
in which Cis the concentration,in mg per mL,of phenoxyacetic acid in the Standard solution,and rUand rSare the phenoxyacetic acid peak responses obtained from the Test solutionand the Standard solution,respectively.Not more than 0.5%is found.
Limit of p-hydroxypenicillin V—
Using the chromatogram of the Assay preparationobtained as directed in the Assay,calculate the percentage of p-hydroxypenicillin Vin the portion of Penicillin Vtaken by the formula:
100rp/rs,
in which rpis the p-hydroxypenicillin Vpeak response;and rsis the sum of the p-hydroxypenicillin Vand penicillin Vpeak responses:not more than 5.0%is found.
Assay—
Mobile phase—
Prepare a suitable filtered and degassed mixture of water,acetonitrile,and glacial acetic acid (650:350:5.75).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Penicillin V Potassium RSin Mobile phaseto obtain a solution having a known concentration of about 2.5mg per mL.
Assay preparation—
Transfer about 125mg of Penicillin V,accurately weighed,to a 50-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Resolution solution—
Prepare a solution in Mobile phasecontaining about 2.5mg of penicillin Gpotassium and 2.5mg of penicillin Vpotassium per mL.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4-mm ×30-cm column that contains packing L1.The flow rate is about 1mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.8for penicillin Gand 1.0for penicillin V,the column efficiency determined from the penicillin Vpeak is not less than 1800theoretical plates,and the resolution,R,between penicillin Gand penicillin Vis not less than 3.0.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major penicillin Vpeaks and any p-hydroxypenicillin Vpeaks with a retention time of about 0.4relative to that of the main penicillin Vpeak.Calculate the quantity,in USP Penicillin V Units,in each mg of the Penicillin Vtaken by the formula:
50(CP/WU)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Penicillin V Potassium RSin the Standard preparation,Pis the designated potency,in USP Penicillin V Units per mg,of USP Penicillin V Potassium RS,WUis the weight,in mg,of Penicillin Vtaken to prepare the Assay preparation,and rUand rSare the sums of the p-hydroxypenicillin Vand penicillin Vpeak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1497
Phone Number:1-301-816-8335