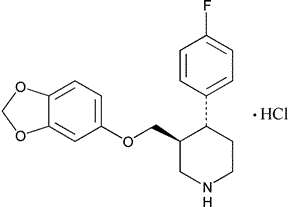
Paroxetine Hydrochloride
C19H20FNO3·HCl
365.83
Piperidine,3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-,hydrochloride,(3S-trans)-.
(-)-(3S,4R)-4-(p-Fluorophenyl)-3-[(3,4-methylenedioxy)phenoxy]methyl]piperidine hydrochloride [78246-49-8].
Piperidine,3-[(1,3-benzodioxol-5-yloxy)methyl]-4-(4-fluorophenyl)-,hydrochloride,(3S-trans)-.
(-)-(3S,4R)-4-(p-Fluorophenyl)-3-[(3,4-methylenedioxy)phenoxy]methyl]piperidine hydrochloride [78246-49-8].
Hemihydrate 374.83
»Paroxetine Hydrochloride is anhydrous or contains one-half molecule of water of hydration.It contains not less than 98.5percent and not more than 102.0percent of C19H20FNO3·HCl,calculated on the anhydrous and solvent-free basis.
Packaging and storage—
Preserve the anhydrous form in tight containers.Preserve the hemihydrate form in well-closed containers.Store between 15 and 30
and 30 .
.
Labeling—
Label it to indicate whether it is the anhydrous or the hemihydrate form.Label it to indicate with which impurity tests the article complies.
USP Reference standards á11ñ—
USP Paroxetine Hydrochloride RS.USP Paroxetine Related Compound A RS.USP Paroxetine Related Compound B RS.USP Paroxetine Related Compound C RS.USP Paroxetine Related Compound E RS.USP Paroxetine Related Compound F RS.USP Paroxetine Related Compound G RS.
Identification—
A:Infrared Absorption á197Mñ—
Test specimen—
Dissolve a suitable portion of Paroxetine Hydrochloride in a solution of water in isopropyl alcohol (1in 10),heat to 70 to dissolve,recrystallize,and dry the residue under vacuum at 50
to dissolve,recrystallize,and dry the residue under vacuum at 50 for 3hours.
for 3hours.
Standard specimen:
a similar preparation of USP Paroxetine Hydrochloride RS.
B:
Asolution (1in 100)in a mixture of methanol and water (1:1)meets the requirements of the test for Chloride á191ñ.
Water,Method Iá921ñ:
not more than 1.0%for the anhydrous form and between 2.2%and 2.8%for the hemihydrate form.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.002%.
Limit of related compound C—
Mobile phase—
Prepare a mixture of n-hexane,alcohol,water,and trifluoroacetic acid (900:100:2:2).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Diluent:
a mixture of alcohol and n-hexane (1:1).
Standard solution—
Dissolve an accurately weighed quantity of USP Paroxetine Related Compound C RS,and dilute quantitatively,and stepwise if necessary,with Diluentto obtain a solution having a known concentration of about 1mg per mL.
Test solution—
Transfer about 125mg of Paroxetine Hydrochloride,accurately weighed,to a 25-mLvolumetric flask,dissolve in and dilute with Diluentto volume,and mix.
System suitability solution—
Dilute known volumes of the Test solutionand the Standard solution with Diluentto obtain a solution having known concentrations of about 0.1mg per mLof each of Paroxetine Hydrochloride and of USP Paroxetine Related Compound C RS.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 295-nm detector and a 4.6-mm ×25-cm column that contains packing L51.The flow rate is about 1.0mLper minute,and the column temperature is maintained at 30 .Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times for paroxetine and paroxetine related compound Cis 1.0and about 0.6,respectively;the resolution,R,between paroxetine and paroxetine related compound Cis not less than 2.0;and the tailing factor for paroxetine related compound Cis not greater than 2.0.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%for the paroxetine related compound C.
.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times for paroxetine and paroxetine related compound Cis 1.0and about 0.6,respectively;the resolution,R,between paroxetine and paroxetine related compound Cis not less than 2.0;and the tailing factor for paroxetine related compound Cis not greater than 2.0.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%for the paroxetine related compound C.
Procedure—
Separately inject equal volumes (about 5µL)of the Standard solution and the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the percentage of paroxetine related compound Cin the portion of Paroxetine Hydrochloride taken by the formula:
2500(C/W)(ri/rS),
in which Cis the concentration,in mg per mL,of USP Paroxetine Related Compound C RSin the Standard solution;Wis the weight,in mg,of Paroxetine Hydrochloride,on the anhydrous basis,used to prepare the Test solution;and ri and rSare the peak areas for paroxetine related compound Cin the Test solutionand the Standard solution,respectively:not more than of 0.1%of paroxetine related compound Cis found.
Limit of 1-methyl-4-(p-fluorophenyl)-1,2,3,6-tetrahydropyridine—
Solution A—
Prepare a filtered and degassed mixture of acetonitrile and trifluoroacetic acid (1000:1).
Solution B—
Prepare a filtered and degassed mixture of water and trifluoroacetic acid (1000:1).
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of USP Paroxetine Related Compound E RSin a mixture of Solution Band Solution A(7:3),and dilute quantitatively,and stepwise if necessary,to obtain a solution having a known concentration of 1-methyl-4-(p-fluorophenyl)-1,2,3,6-tetrahydropyridine of about 100ng per mL.
Test solution—
Transfer about 20mg of Paroxetine Hydrochloride,accurately weighed,to a suitable flask,add 1.0mLof a mixture of Solution Band Solution A(7:3),and shake to dissolve.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a tandem mass spectrophotometric detector,monitoring the mass-to-charge ratio of 44arising from the fragmentation of mass-to-charge ratio of 192,and a 2.0-mm ×25-cm column that contains base-deactivated packing L1.The flow rate is about 0.15mLper minute.The collision-induced disassociation sector is filled with sufficient argon gas to produce -20eVcollisions.Adjust the argon gas pressure as necessary.The chromatograph is programmed as follows.
Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the signal-to-noise ratio for the analyte response at a mass-to-charge ratio of 44is not less than 5;and the relative standard deviation for replicate injections is not more than 5.0%.[NOTE—Alarge peak due to paroxetine is observed at about 10minutes in this system.Divert the flow of eluate from the mass spectrometer at about 10minutes after injection.]
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 30 | 70 | equilibration |
| 0–10 | 30 | 70 | isocratic |
| 10–10.5 | 30®90 | 70®10 | linear gradient |
| 10.5–20 | 90 | 10 | isocratic |
| 20–20.5 | 90®30 | 10®70 | linear gradient |
| 20.5–30 | 30 | 70 | isocratic |
Procedure—
Separately inject equal volumes (about 100µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the amount,in ng,of 1-methyl-4-(p-fluorophenyl)-1,2,3,6-tetrahydropyridine in the portion of Paroxetine Hydrochloride taken by the formula:
CI(rU/rS),
in which Cis the concentration,in mg per mL,of USP Paroxetine Related Compound E RSin the Standard solution;Iis the amount,in ng,of 1-methyl-4-(p-fluorophenyl)-1,2,3,6-tetrahydropyridine in each mg of USP Paroxetine Related Compound E RSin the Standard solution;and rUand rSare the peak responses for 1-methyl-4-(p-fluorophenyl)-1,2,3,6-tetrahydropyridine obtained from the Test solutionand the Standard solution,respectively:not more than 20ng is found (0.0001%).
Chromatographic purity—
[NOTE—Perform all related impurities methods unless the manufacturer has assurance,based on knowledge of the manufacturing process,that one of the tests is not relevant to their material.]
TEST1—
Solution A—
Prepare a filtered and degassed mixture of water,tetrahydrofuran,and trifluoroacetic acid (180:20:1).
Solution B—
Prepare a filtered and degassed mixture of acetonitrile,tetrahydrofuran,and trifluoroacetic acid (180:20:1).
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Diluent:
a mixture of water and tetrahydrofuran (9:1).
Standard solution—
Dissolve an accurately weighed quantity of USP Paroxetine Hydrochloride RS,and dilute quantitatively,and stepwise if necessary,with Diluent to obtain a solution having a known concentration of about 1µg per mL.
System suitability solution—
Dissolve,by sonication if necessary,suitable quantities of USP Paroxetine Related Compound A RSand USP Paroxetine Related Compound B RSinDiluentto obtain a solution having known concentrations of about 0.01mg of each USP Reference Standard per mL.
Test solution—
Transfer about 25mg of Paroxetine Hydrochloride,accurately weighed,to a 25-mLvolumetric flask,dissolve in 20mLofDiluent,sonicate,dilute with Diluentto volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 285-nm detector and a 4.6-mm ×25-cm column that contains packing L7.The flow rate is about 1mLper minute.The column temperature is maintained at 40 .The chromatograph is programmed as follows.
.The chromatograph is programmed as follows.
Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 1.1for paroxetine related compound B,and 1.0for paroxetine related compound A;the resolution,R,between paroxetine related compound Aand paroxetine related compound Bis not less than 2.0;the tailing factor of the paroxetine related compound Apeak is between 0.8and 2.0;and the relative standard deviation for replicate injections is not more than 2.0%for paroxetine related compound A.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 80 | 20 | equilibration |
| 0–30 | 80 | 20 | isocratic |
| 30–50 | 80®20 | 20®80 | linear gradient |
| 50–60 | 20 | 80 | isocratic |
| 60–70 | 20®80 | 80®20 | linear gradient |
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solution,the Test solution,and the Diluentinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the percentage of each impurity in the portion of Paroxetine Hydrochloride taken by the formula:
2500(C/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Paroxetine Hydrochloride RSin the Standard solution;Wis the weight,in mg,of Paroxetine Hydrochloride,on the anhydrous basis,used to prepare the Test solution;rUis the peak area of each impurity in the Test solution,excluding the peaks obtained in the chromatogram of the Diluent;and rSis the peak area of paroxetine obtained in the Standard solution:not more than of 0.3%of any peak at a retention time of paroxetine related compound Bis found;not more that 0.1%of any other individual impurity is found;and not more than 1.0%of total impurities is found.
TEST2—
Phosphate buffer—
Dissolve 3.4g of monobasic potassium phosphate and 3.4g of tetrabutylammonium hydrogen sulfate in 1.0Lof water.
Solution A—
Prepare a filtered and degassed mixture of Phosphate buffer and acetonitrile (98:2).
Solution B—
Prepare a filtered and degassed mixture of Phosphate buffer and acetonitrile (6:4).
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Diluent:
a mixture of Phosphate bufferand acetonitrile (9:1).
Standard solution—
Dissolve an accurately weighed quantity of USP Paroxetine Hydrochloride RS,USP Paroxetine Related Compound B RS,USP Paroxetine Related Compound F RS,and USP Paroxetine Related Compound G RSin Diluent,and dilute quantitatively,and stepwise if necessary,with Diluentto obtain a solution having known concentrations of about 4µg per mL,10µg per mL,10µg per mL,and 4µg per mL,respectively.
Identification solution—
Dissolve an accurately weighed quantity of Paroxetine Hydrochloride,USP Paroxetine Related Compound B RS,USP Paroxetine Related Compound F RS,and USP Paroxetine Related Compound G RSin Diluent to obtain a solution having known concentrations of about 2mg per mL,10µg per mL,10µg per mL,and 4µg per mL,respectively.
Test solution—
Transfer about 25mg of Paroxetine Hydrochloride,accurately weighed,to a 50-mLvolumetric flask,dissolve in and dilute with Diluent to volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 210-nm detector and a 3.9-mm ×15-cm column that contains packing L1.The flow rate is about 1.0mLper minute.The chromatograph is programmed as follows.
Chromatograph theIdentification solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.91for paroxetine related compound B,about 0.96for paroxetine related compound F,1.0for paroxetine,and about 1.34for paroxetine related compound G.Chromatograph the Standard solution,and record the peak responses as directed forProcedure:the relative standard deviation for replicate injections is not more than 10.0%for the paroxetine related compound B,paroxetine related compound F,paroxetine hydrochloride,and paroxetine related compound Gpeaks.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 100 | 0 | equilibration |
| 0–5 | 100 | 0 | isocratic |
| 5–70 | 100®40 | 0®60 | linear gradient |
| 70–90 | 40®0 | 60®100 | linear gradient |
| 90–95 | 0 | 100 | isocratic |
| 95–95.1 | 0®100 | 100®0 | linear gradient |
| 95.1–110 | 100 | 0 | re-equilibration |
Procedure—
Separately inject equal volumes (about 25µL)of the Standard solution and Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the percentage of paroxetine related compound B,paroxetine related compound F,and paroxetine related compound Gin the portion of Paroxetine Hydrochloride taken by the formula:
5000(C/W)(ri/rS),
in which Cis the concentration,in mg per mL,of the appropriate USP Reference Standard in the Standard solution;Wis the weight,in mg,of Paroxetine Hydrochloride,on the anhydrous basis,used to prepare the Test solution;and riand rSare the peak areas for the corresponding impurity in the Test solution and the Standard solution,respectively:not more than of 0.5%of paroxetine related compound Bis found;not more than 0.2%of paroxetine related compound Fis found;and not more than 0.2%of paroxetine related compound Gis found.Calculate the percentage of any unknown impurity in the portion of Paroxetine Hydrochloride taken by the formula:
5000(C/W)(ri/rS),
in which Cis the concentration,in mg per mL,of USP Paroxetine Hydrochloride RSin the Standard solution;Wis the weight,in mg,of Paroxetine Hydrochloride,on the anhydrous basis,used to prepare the Test solution;riis the peak area for any unknown impurity in the Test solution;and rSis the peak area of paroxetine in the Standard solution:not more than of 0.1%of any single unknown impurity is found,and not more than 1.0%of total impurities is found.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Assay—
Acetate buffer—
Prepare a 0.05Msolution of ammonium acetate in water,adjust with glacial acetic acid to a pHof 4.5,mix,and filter.
Mobile phase—
Prepare a filtered and degassed mixture of Acetate buffer,acetonitrile,and triethylamine (60:40:1).Adjust with glacial acetic acid to a pHof 5.5.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
System suitability solution—
Dissolve suitable quantities of USP Paroxetine Related Compound B RSand USP Paroxetine Hydrochloride RSin water to obtain a solution having known concentrations of about 0.5mg of each USP Reference Standard per mL.
Standard preparation—
Dissolve an accurately weighed quantity of USP Paroxetine Hydrochloride RSin water,and dilute quantitatively,and stepwise if necessary,with water to obtain a solution having a known concentration of about 0.5mg per mL.
Assay preparation—
Transfer about 50mg of Paroxetine Hydrochloride,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 295-nm detector and a 4.6-mm ×25-cm column that contains packing L13.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.9for paroxetine related compound Band 1.0for paroxetine;the resolution,R,between paroxetine related compound Band paroxetine is not less than 2.0;the column efficiency determined from the paroxetine peak is not less than 3000theoretical plates;the tailing factor for the paroxetine peak is not more than 1.6;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C19H20FNO3·HCl in the portion of Paroxetine Hydrochloride taken by the formula:
100C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Paroxetine Hydrochloride RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Salvador C.Salado,M.S.,Scientist and Latin American Liaison
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1474
Pharmacopeial Forum:Volume No.30(4)Page 1282
Phone Number:1-301-816-8165