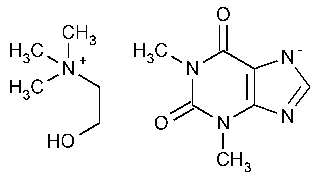
Oxtriphylline
Ethanaminium,2-hydroxy-N,N,N-trimethyl-,salt with 3,7-dihydro-1,3-dimethyl-1H-purine-2,6-dione.
Choline salt with theophylline (1:1) [4499-40-5].
»Oxtriphylline contains not less than 61.7percent and not more than 65.5percent of anhydrous theophylline (C7H8N4O2),calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Identification—
Dissolve about 1g in 20mLof water,and use the solution for the following tests.
A:
To 10mLof the test solution add 5mLof mercuric-potassium iodide TS:a pale yellow precipitate is formed (presence of choline).
B:
To 10mLof the test solution add 5drops of 6Nammonium hydroxide and 5mLof silver nitrate TS:a gelatinous precipitate is formed,and it coagulates on heating (presence of theophylline).
Loss on drying á731ñ—
Dry it at 80 for 4hours:it loses not more than 1.0%of its weight.
for 4hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.3%.
Chloride á221ñ—
A0.50-g portion shows no more chloride than corresponds to 0.15mLof 0.020Nhydrochloric acid (0.02%).
Ordinary impurities á466ñ—
Test solution:
a mixture of chloroform,alcohol,and formic acid (88:10:2).
Standard solution:
a mixture of chloroform,alcohol,and formic acid (88:10:2).
Eluant:
a mixture of chloroform,alcohol,and formic acid (88:10:2).
Visualization:
1.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Choline content—
Dissolve about 900mg of Oxtriphylline,accurately weighed,in 50mLof water,and add 4drops of a solution prepared by dissolving 30mg of methyl red in 100mLof methanol,adding 15mLof methylene blue solution (1in 1000),and mixing.Mix,and titrate with 0.1Nsulfuric acid VSto a purple endpoint.Each mLof 0.1Nsulfuric acid is equivalent to 12.12mg of C5H15NO2.The content of choline (C5H15NO2)is between 652mg and 693mg per g of C7H8N4O2found in the Assay.Retain the final solution for the Assay for theophylline.
Assay for theophylline—
To the solution retained in the Choline contentadd 35mLof silver nitrate TS,swirl gently to promote complete precipitation,and titrate with 0.1Nsodium hydroxide VSto a green endpoint.Each mLof 0.1Nsodium hydroxide is equivalent to 18.02mg of C7H8N4O2.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 1432
Phone Number:1-301-816-8379