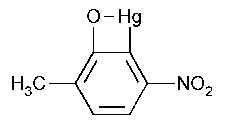
Nitromersol
7-Oxa-8-mercurabicyclo[4.2.0]octa-1,3,5-triene,5-methyl-2-nitro-.
5-Methyl-2-nitro-7-oxa-8-mercurabicyclo[4.2.0]octa-1,3,5-triene [133-58-4].
»Nitromersol,dried at 105 for 2hours,contains not less than 98.0percent and not more than 100.5percent of C7H5HgNO3.
for 2hours,contains not less than 98.0percent and not more than 100.5percent of C7H5HgNO3.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Asolution (1in 1000)in 1Nsodium hydroxide possesses a reddish-orange color.The addition of 3Nhydrochloric acid to this solution causes the color to disappear and a yellowish,flocculent precipitate to form.
B:
To a solution prepared by dissolving 250mg of Nitromersol in 2.5mLof 1Nsodium hydroxide and diluting with water to 20mLadd about 3mLof 3Nhydrochloric acid:a yellowish precipitate is formed.Upon filtration,the filtrate is nearly colorless or slightly yellow.Retain the filtrate for the test for Mercury ions.Dissolve the precipitate in 20mLof water to which 2.5mLof 1Nsodium hydroxide has been added,add 0.5g of sodium hydrosulfite,and heat to boiling:a heavy deposit of metallic mercury is formed.
Loss on drying á731ñ—
Dry it at 105 for 2hours:it loses not more than 1.0%of its weight.
for 2hours:it loses not more than 1.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Mercury ions—
To the filtrate obtained in Identificationtest Badd an equal volume of hydrogen sulfide TS:no darkening in color is produced,although a small amount of a flocculent,light yellow precipitate may form.
Alkali-insoluble substances—
Add 7mLof 1Nsodium hydroxide to 1.0g of Nitromersol,then dilute with water to 20mL.The resulting solution,upon standing in a glass-stoppered vessel in the dark for 24hours,shows no more than a slight amount of insoluble material.Collect the insoluble residue,if any,in a tared filter crucible,wash the residue with warm water,and dry at 105 for 1hour:the weight of the insoluble material does not exceed 1mg (0.1%).
for 1hour:the weight of the insoluble material does not exceed 1mg (0.1%).
Uncombined nitrocresol—
Shake 500mg of Nitromersol with 50mLof benzene,filter,evaporate the filtrate in a tared dish to dryness,and dry the residue at 80 for 2hours:the weight of the residue does not exceed 5mg (1%).
for 2hours:the weight of the residue does not exceed 5mg (1%).
Assay—
Weigh accurately about 200mg of Nitromersol,previously ground to a fine powder and dried,and transfer to a 500-mL Kjeldahl flask.Add 15mLof sulfuric acid,digest cautiously with occasional swirling over a flame until the solution becomes a clear,light yellowish brown,cool,and add,dropwise,enough 30percent hydrogen peroxide to decolorize the solution.Digest for 2to 3minutes,adding more hydrogen peroxide,if necessary,to produce a colorless solution.Cool,dilute with water to about 100mL,and add potassium permanganate TSuntil a permanent pink color persists on heating.Then add hydrogen peroxide TS,dropwise,until the color is completely discharged.Cool,and add 5mLof nitric acid which has been diluted with 10mLof water.Add 5mLof ferric ammonium sulfate TS,and titrate with 0.1Nammonium thiocyanate VS.Each mLof 0.1Nammonium thiocyanate is equivalent to 17.59mg of C7H5HgNO3.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 1387
Phone Number:1-301-816-8394