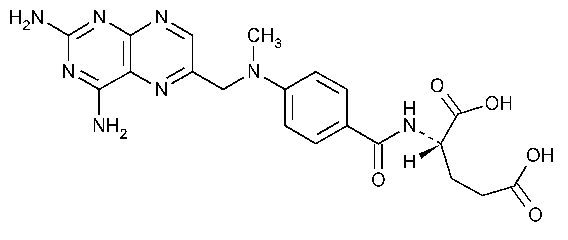
Methotrexate
L-Glutamic acid,N-[4[[-(2,4-diamino-6-pteridinyl)methyl]-methylamino]benzoyl]-.
L-(+)-N-[p-[[(2,4-Diamino-6-pteridinyl)methyl]methylamino]-benzoyl]glutamic acid [59-05-2].
»Methotrexate is a mixture of 4-amino-10-methylfolic acid and closely related compounds.It contains not less than 98.0percent and not more than 102.0percent of C20H22N8O5,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Infrared Absorption á197Kñ—Do not dry specimens.
Specific rotation á781Sñ:
between +19 and +24
and +24 ,2-dm polarimeter tube being used.
,2-dm polarimeter tube being used.
Test solution:
10mg per mL,in 0.05Msodium carbonate.
Water,Method Iá921ñ:
not more than 12.0%.
Residue on ignition á281ñ:
not more than 0.1%.
Chromatographic purity—
pH6.0Buffer solution
,Mobile phase,System suitability solution,and Chromatographic system—Proceed as directed in the Assay.
Standard preparation—
Dissolve an accurately weighed quantity of USP Methotrexate RSin Mobile phaseto obtain a solution having a known concentration of about 5µg per mL.
Test preparation—
Transfer about 100mg of Methotrexate,accurately weighed,to a 100-mLvolumetric flask,dissolve in Mobile phase,with the aid of sonication or shaking if necessary,dilute with Mobile phaseto volume,and mix.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Separately inject equal volumes (about 10µL)of the Standard preparationand the Test preparationinto the chromatograph,and allow the Test preparationto elute for not less than three times the retention time of methotrexate.Record the chromatograms,and measure the peak responses.The sum of all of the peak responses,other than that of methotrexate,is not more than four times the methotrexate response from the Standard preparation(2.0%),and no single peak response is greater than that of the methotrexate response from the Standard preparation(0.5%).
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent:
dimethyl sulfoxide.
Assay—
pH6.0Buffer solution—
Prepare a mixture of 0.2Mdibasic sodium phosphate and 0.1Mcitric acid (630:370).Adjust if necessary with 0.1Mcitric acid or 0.2Mdibasic sodium phosphate to a pHof 6.0.
Mobile phase—
Prepare a filtered and degassed solution of pH6.0buffer solutionand acetonitrile (90:10).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Methotrexate RSin Mobile phaseto obtain a solution having a known concentration of about 100µg per mL.
Assay preparation—
Transfer about 25mg of Methotrexate,accurately weighed,to a 250-mLvolumetric flask,dissolve in Mobile phase,dilute with Mobile phaseto volume,and mix.
System suitability solution—
Prepare a solution in Mobile phasecontaining about 0.1mg per mLeach of USP Methotrexate RSand folic acid.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 302-nm detector and a 4.6-mm ×25-cm column that contains packing L1.The flow rate is about 1.2mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.35for folic acid and 1.0for methotrexate,the resolution,R,between the folic acid and methotrexate peaks is not less than 8.0,and the relative standard deviation for replicate injections is not more than 2.5%for methotrexate.
Procedure—
Separately inject equal volumes (about 10µL)of the Assay preparationand the Standard preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of C20H22N8O5in the portion of Methotrexate taken by the formula:
(0.25C)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Methotrexate RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 1248
Phone Number:1-301-816-8389