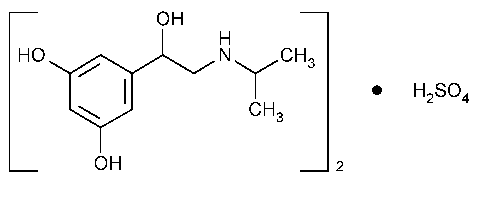
Metaproterenol Sulfate
1,3-Benzenediol,5-[1-hydroxy-2-(1-methylethyl)amino]ethyl-,(±)-,sulfate (2:1)(salt).
(±)-3,5-Dihydroxy-a-[(isopropylamino)methyl]benzyl alcohol sulfate (2:1) [5874-97-5].
»Metaproterenol Sulfate contains not less than 98.0percent and not more than 102.0percent of (C11H17NO3)2·H2SO4,calculated on the anhydrous,isopropyl alcohol-free,and methanol-free basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:
Infrared Absorption á197Kñ.
B:
To a solution of 10mg in 1mLof water add 1drop of ferric chloride TS:a violet color is produced.
C:
It responds to the tests for Sulfate á191ñ.
pHá791ñ:
between 4.0and 5.5,in a solution containing 100mg per mL.
Water,Method Iá921ñ:
not more than 2.0%.
Residue on ignition á281ñ:
not more than 0.1%.
Heavy metals,Method IIá231ñ:
0.001%.
Iron á241ñ—
Dissolve 2.0g in 45mLof water,add 2mLof hydrochloric acid,and mix:the limit is 5ppm.
Limit of metaproterenone sulfate—
Its absorptivity (see Spectrophotometry and Light-Scattering á851ñ)at 328nm,determined in an aqueous solution containing 9.0mg per mL,is not more than 0.009(0.1%).
Isopropyl alcohol and methanol—
Isopropyl alcohol standard solution—
Transfer about 0.3g of isopropyl alcohol,accurately weighed,to a 100-mLvolumetric flask containing about 10mLof water,dilute with water to volume,and mix.Pipet 10mLof the resulting solution into a 100-mLvolumetric flask,add about 85mLof pyridine,mix,and allow to stand for 1hour.Dilute with pyridine to volume,and mix.Pipet 5mLof this solution to a 50-mLvolumetric flask,dilute with pyridine to volume,and mix.The solution so obtained contains about 30µg of isopropyl alcohol per mL.
Methanol standard solution—
Prepare as directed for Isopropyl alcohol standard solution,using about 0.1g of methanol,accurately weighed.The resulting solution contains about 10µg of methanol per mL.
Test preparation—
Transfer about 1g of Metaproterenol Sulfate,accurately weighed,to a 100-mLvolumetric flask,dissolve in about 2mLof water,dilute with pyridine to volume,and mix.
Chromatographic system—
The gas chromatograph is equipped with a flame-ionization detector and contains a 2-m ×2-mm column packed with 0.1%liquid phase G25on 80-to 100-mesh support S7.The injection port is maintained at a temperature of about 150 ;the column is programmed for 2minutes at 40
;the column is programmed for 2minutes at 40 ,to increase at a rate of about 15
,to increase at a rate of about 15 per minute to 200
per minute to 200 ,and for 10minutes at 200
,and for 10minutes at 200 ;the detector is maintained at about 250
;the detector is maintained at about 250 ;and helium is used as the carrier gas at a flow rate of about 15mLper minute.
;and helium is used as the carrier gas at a flow rate of about 15mLper minute.
Procedure—
Inject equal volumes (about 2µL)of the Test preparation,the Isopropyl alcohol standard solution,and the Methanol standard solutionsuccessively into the gas chromatograph.Measure the responses of the isopropyl alcohol peak and the methanol peak in each chromatogram.Determine the quantities,in mg,of isopropyl alcohol and methanol in the portion of Metaproterenol Sulfate taken by the formula:
0.1C(rU/rS),
in which Cis the concentration,in µg per mL,of isopropyl alcohol or methanol in the Isopropyl alcohol standard solutionor the Methanol standard solution;and rUand rSare the responses of the respective analytes in the Test preparationand of the corresponding Isopropyl alcohol standard solutionor Methanol standard solution:not more than 0.3%of isopropyl alcohol and not more than 0.1%of methanol are found.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
Mobile phase—
Dissolve 11.9g of anhydrous dibasic sodium phosphate in water to make 1000mLof solution,and mix (Solution A).Dissolve 9.1g of monobasic potassium phosphate in water to make 1000mLof solution,and mix (Solution B).Mix 735mLof Solution Aand 140mLof Solution B,add 125mLof methanol,and mix.Filter and degas this solution before use.
Standard preparation—
Dissolve an accurately weighed quantity of USP Metaproterenol Sulfate RSin 0.01Nhydrochloric acid to obtain a solution having a known concentration of about 2mg per mL.
Assay preparation—
Transfer about 100mg of Metaproterenol Sulfate,accurately weighed,to a 50-mLvolumetric flask,dilute with 0.01Nhydrochloric acid to volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 278-nm detector and a 4.6-mm ×5-cm guard column that contains packing L7and a 4.6-mm ×25-cm analytical column that contains 10-µm packing L7.The flow rate is about 2mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the column efficiency determined from the analyte peak is not less than 500theoretical plates,the tailing factor for the analyte peak is not more than 3.0,and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of (C11H17NO3)2·H2SO4in the portion of Metaproterenol Sulfate taken by the formula:
50C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Metaproterenol Sulfate RSin the Standard preparation,and rUand rSare the peak responses from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 1227
Phone Number:1-301-816-8379