Maltodextrin
»Maltodextrin is a nonsweet,nutritive saccharide mixture of polymers that consists of D-glucose units,with a Dextrose Equivalent less than 20.It is prepared by the partial hydrolysis of a food grade starch with suitable acids and/or enzymes.It may be physically modified to improve its physical and functional characteristics.
Packaging and storage—
Preserve in tight containers,or in well-closed containers at a temperature not exceeding 30 and a relative humidity not exceeding 50%.
and a relative humidity not exceeding 50%.
Microbial limits á61ñ—
It meets the requirements of the tests for absence of Salmonellaspecies and Escherichia coli.
pHá791ñ:
between 4.0and 7.0,in a 1in 5solution in carbon dioxide-free water.
Loss on drying á731ñ—
Dry it at 105 for 2hours in a forced-air oven:it loses not more than 6.0%of its weight.
for 2hours in a forced-air oven:it loses not more than 6.0%of its weight.
Residue on ignition á281ñ:
not more than 0.5%.
Heavy metals,Method IIá231ñ:
5ppm.
Protein—
Transfer about 10g of Maltodextrin,accurately weighed,to an 800-mL Kjeldahl flask,and add 10g of anhydrous potassium sulfate or sodium sulfate,300mg of copper selenite or mercuric oxide,and 60mLof sulfuric acid.Gently heat the mixture,keeping the flask inclined at about a 45 angle,and after frothing has ceased,boil briskly until the solution has remained clear for about 1hour.Cool,very cautiously add about 50mLof water while swirling to dissipate the resulting heat.Add an additional 150to 250mLof water,mix,and cool again.Cautiously pour about 75mL(or enough to make the mixture strongly alkaline)of sodium hydroxide solution (2in 5)down the inside of the flask so that it forms a layer under the acid solution,and then add a few pieces of granular zinc.Immediately connect the flask to a distillation apparatus consisting of a Kjeldahl connecting bulb and a condenser,the delivery tube of which extends well beneath the surface of an accurately measured excess of 0.1Nsulfuric acid contained in a 500-mLflask.Gently rotate the contents of the Kjeldahl flask to mix,and distill until all ammonia has passed into the absorbing acid solution (about 250mLof distillate).To the receiving flask add 0.25mLof methyl red-methylene blue TS,and titrate the excess acid with 0.1Nsodium hydroxide.Perform a blank determination,substituting pure sucrose or dextrose for the test specimen,and make any necessary correction.Each mLof 0.1Nsulfuric acid consumed is equivalent to 1.401mg of nitrogen (N).Calculate the percentage of Nin the specimen taken,and then calculate the percentage of protein by multiplying the percentage of Nby 6.25.The limit is 0.1%.
angle,and after frothing has ceased,boil briskly until the solution has remained clear for about 1hour.Cool,very cautiously add about 50mLof water while swirling to dissipate the resulting heat.Add an additional 150to 250mLof water,mix,and cool again.Cautiously pour about 75mL(or enough to make the mixture strongly alkaline)of sodium hydroxide solution (2in 5)down the inside of the flask so that it forms a layer under the acid solution,and then add a few pieces of granular zinc.Immediately connect the flask to a distillation apparatus consisting of a Kjeldahl connecting bulb and a condenser,the delivery tube of which extends well beneath the surface of an accurately measured excess of 0.1Nsulfuric acid contained in a 500-mLflask.Gently rotate the contents of the Kjeldahl flask to mix,and distill until all ammonia has passed into the absorbing acid solution (about 250mLof distillate).To the receiving flask add 0.25mLof methyl red-methylene blue TS,and titrate the excess acid with 0.1Nsodium hydroxide.Perform a blank determination,substituting pure sucrose or dextrose for the test specimen,and make any necessary correction.Each mLof 0.1Nsulfuric acid consumed is equivalent to 1.401mg of nitrogen (N).Calculate the percentage of Nin the specimen taken,and then calculate the percentage of protein by multiplying the percentage of Nby 6.25.The limit is 0.1%.
Sulfur dioxide—
Hydrogen peroxide solution—
Dilute 30percent hydrogen peroxide with water to obtain a 3%solution.Just before use,add 3drops of methyl red TS,and neutralize to a yellow endpoint with 0.01Nsodium hydroxide.Do not exceed the endpoint.
Nitrogen—
Use high-purity nitrogen,with a flow regulator that will maintain a flow of 200±10mLper minute.Guard against the presence of oxygen by passing the nitrogen through a scrubber,such as alkaline pyrogallol,prepared as follows.Add 4.5g of pyrogallol to a gas-washing bottle,purge the bottle with nitrogen for 3minutes,and add a solution containing 85mLof water and 65g of potassium hydroxide,while maintaining an atmosphere of nitrogen in the bottle.
Apparatus—
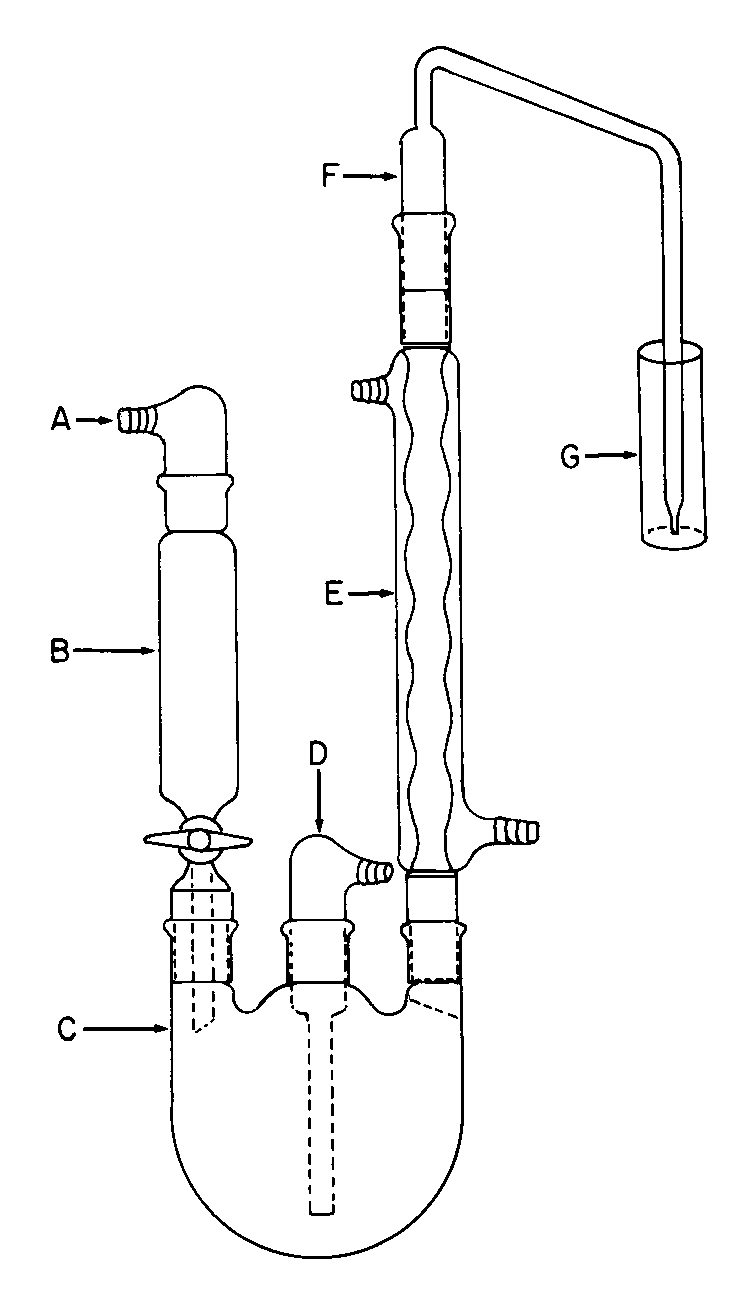
The apparatus (see Fig.1)is designed to effect the selective transfer of sulfur dioxide from the specimen in boiling aqueous hydrochloric acid to the Hydrogen peroxide solution.The backpressure is limited to the unavoidable pressure due to the height of the Hydrogen peroxide solutionabove the tip of the bubbler,F.Keeping the backpressure as low as possible reduces the likelihood that sulfur dioxide will be lost through leaks.Preboil vinyl and silicone tubing.Apply a thin film of stopcock grease to the sealing surfaces of all of the joints except the joint between the separatory funnel and the flask,and clamp the joints to ensure tightness.The separatory funnel,B,has a capacity of 100mLor greater.The inlet adapter,A,with a hose connector provides a means of applying headpressure over the solution.[NOTE—Apressure-equalizing dropping funnel is not recommended because condensate,which may contain sulfur dioxide,is deposited in the funnel and the side arm.]
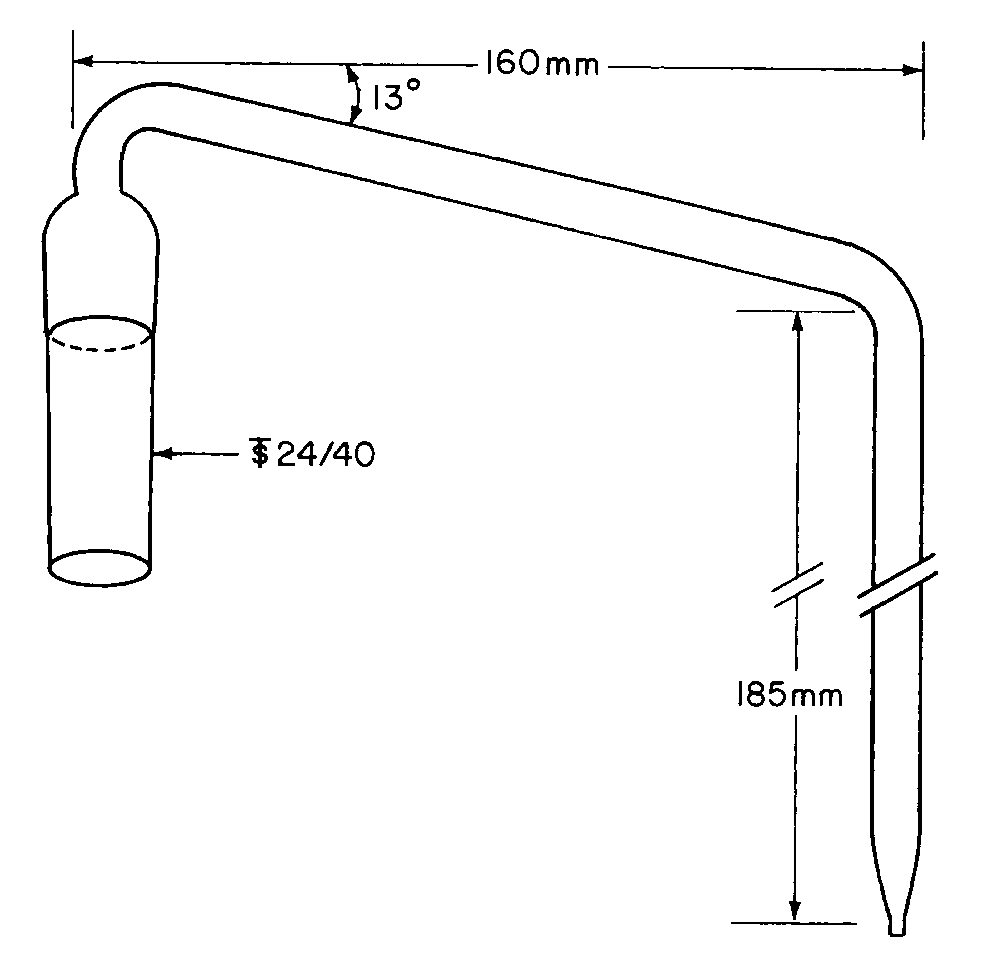
The round-bottom flask,C,is a 1000-mLflask with three 24/40tapered joints.The gas inlet tube,D,is long enough to permit introduction of the nitrogen within 2.5cm of the bottom of the flask.The Allihn condenser,E,has a jacket length of 300mm.The bubbler,F,(see Fig.2)is fabricated from glass according to the dimensions given in Figure 2.The Hydrogen peroxide solutionis contained in a vessel,G,having an inside diameter of about 2.5cm and a depth of about 18cm.Circulate coolant,such as a mixture of water and methanol (4:1)maintained at 5 ,to chill the condenser.
,to chill the condenser.
Procedure—
Position the Apparatusin a heating mantle controlled by a power-regulating device.Add 400mLof water to the flask.Close the stopcock of the separatory funnel,and add 90mLof 4Nhydrochloric acid to the separatory funnel.Begin the flow of nitrogen at a rate of 200±10mLper minute.Start the condenser coolant flow.Add 30mLof Hydrogen peroxide solutionto vessel G.After 15minutes,remove the separatory funnel,and transfer a mixture of 50.0g of Maltodextrin,accurately weighed,and 100mLof alcohol solution (5in 100).Apply stopcock grease to the outer joint of the separatory funnel,return the separatory funnel to the tapered joint flask,and concomitantly resume the nitrogen flow.Apply headpressure above the hydrochloric acid solution in the separatory funnel with a rubber bulb equipped with a valve.Open the stopcock of the separatory funnel to permit the hydrochloric acid solution to flow into the flask.Continue to maintain sufficient pressure above the hydrochloric acid solution to force it into the flask.[NOTE—The stopcock may be temporarily closed,if necessary,to pump up the pressure.]To guard against escape of sulfur dioxide into the separatory funnel,close the stopcock before the last few mLof hydrochloric acid drain out.Apply power to the heating mantle sufficient to cause about 85drops of reflux per minute.After refluxing for 1.75hours,remove vessel G,add 3drops of methyl red TS,and titrate the contents with 0.01Nsodium hydroxide VS,using a 10-mLburet with an overflow tube and a hose connection to a carbon dioxide-absorbing tube,to a yellow endpoint that persists for at least 20seconds.Perform a blank determination,and make any necessary correction (see Titrimetry á541ñ).Calculate the quantity,in µg,of SO2in each g of the Maltodextrin taken by the formula:
1000(32.03)VN/W,
in which 32.03is the milliequivalent weight of sulfur dioxide;Vis the volume,in mL,of titrant consumed;Nis the normality of the titrant;and Wis the weight,in g,of Maltodextrin taken.Not more than 40µg of sulfur dioxide per g of Maltodextrin are found (.004%).
Dextrose equivalent—
Standard solution—
Dissolve an accurately weighed quantity of USP Dextrose RSin water,and dilute quantitatively with water to obtain a solution having a known concentration of about 10mg per mL.
Test solution—
Transfer about 5g of Maltodextrin,accurately weighed,with the aid of hot water to a 100-mLvolumetric flask,cool,add water to volume,and mix.
Procedure—
Transfer 25.0-mLportions of alkaline cupric tartrate TSto each of two boiling flasks.Bring the contents of one flask to boiling within about 2minutes while titrating with Standard solutionto within 0.5mLof the anticipated endpoint.Boil gently for 2minutes.Continue to boil gently,add 2drops of methylene blue solution (1in 100),and complete the titration within 1minute by adding the Standard solutiondropwise or in small increments until the blue color disappears,determined by viewing against a white background in daylight or under equivalent illumination.If more than 0.5mLof the titrant was required after the addition of the indicator,repeat the titration,adding the necessary volume of titrant before adding the indicator.Bring the contents of the second flask to boiling,and similarly titrate with Test solution.Calculate the Dextrose equivalent,on the dried basis,taken by the formula:
[100/(1-0.01A)](CS/CU)(VS/VU),
in which Ais the percentage Loss on drying of the Maltodextrin taken;CUis the concentration,in mg per mL,of Maltodextrin in the Test solution;CSis the concentration,in mg per mL,of USP Dextrose RSin the Standard solution;and VUand VSare the titrated volumes,in mL,of the Test solutionand the Standard solution,respectively.The Dextrose equivalentis less than 20.[NOTE—This is a limit test.For Maltodextrins with lower reducing values,other procedures may give other results.]
Auxiliary Information—
Staff Liaison:Catherine Sheehan,B.Sc.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 3033
Phone Number:1-301-816-8262