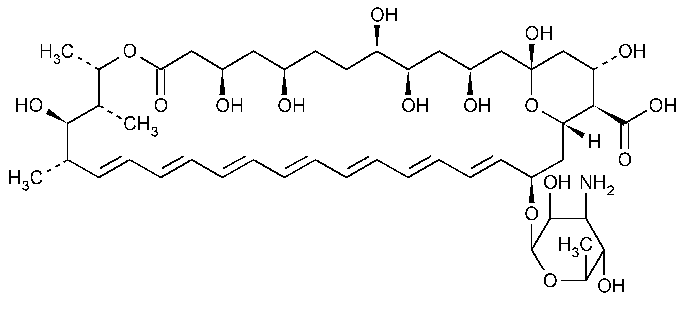
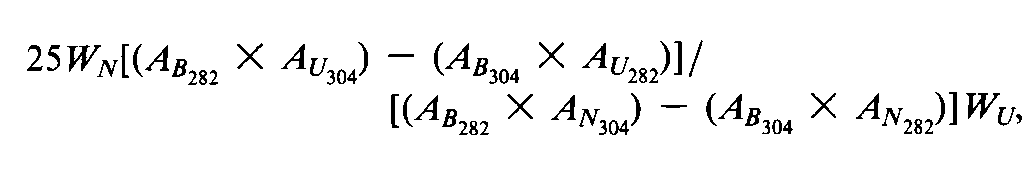Amphotericin B
Amphotericin B.
Amphotericin B.
[1R-(1R*,3S*,5R*,6R*,9R*,11R*,15S*,16R*,17R*,18S*,19E,21E,23E,25E,27E,29E,31E,33R*,35S*,36R*,37S*)]-33-[(3-Amino-3,6-dideoxy-b-D-mannopyranosyl)oxy]-1,3,5,6,9,11,17,37-octahydroxy-15,16,18-trimethyl-13-oxo-14,-39-dioxabicyclo[33.3.1]nonatriaconta-19,21,23,25,27,29,31-heptaene-36-carboxylic acid [1397-89-3].
»Amphotericin Bhas a potency of not less than 750µg of C47H73NO17per mg,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers,and store in a cold place.
Labeling—
Label it to state whether it is intended for use in preparing dermatological and oral dosage forms or parenteral dosage forms.
Identification,Ultraviolet Absorption á197Uñ—
Spectral range 1:
240to 320nm.
Solution 1:
prepared as directed for Test preparationin the Limit of amphotericin A,and compare its absorbance to that of the Amphotericin Bstandard preparation.An extra peak may occur at 304nm in the spectrum of this solution.
Spectral range 2:
320to 400nm.
Solution 2:
prepared as directed for Test preparationin the Limit of amphotericin Aand then diluted with 9volumes of methanol.Compare its absorbance to that of a similar dilution of the Amphotericin Bstandard preparation.
Loss on drying á731ñ—
Dry about 100mg,accurately weighed,in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5mm of mercury at 60 for 3hours:it loses not more than 5.0%of its weight.
for 3hours:it loses not more than 5.0%of its weight.
Residue on ignition á281ñ:
not more than 0.5%,the charred residue being moistened with 2mLof nitric acid and 5drops of sulfuric acid.[NOTE—Amphotericin Bintended for use in preparing dermatological creams,lotions,and ointments,and oral suspensions and capsules,yields not more than 3.0%.]
Limit of amphotericin A—
Test preparation—
Dissolve about 50mg of Amphotericin B,accurately weighed,in 10.0mLof dimethyl sulfoxide in a 50-mLvolumetric flask,dilute with methanol to volume,and mix.Transfer 4.0mLof this solution to a 50-mLvolumetric flask,dilute with methanol to volume,and mix.
Nystatin standard preparation—
Dissolve about 20mg of USP Nystatin RS,accurately weighed,in 40.0mLof dimethyl sulfoxide in a 200-mLvolumetric flask,dilute with methanol to volume,and mix.Transfer 4.0mLof this solution to a 50-mLvolumetric flask,dilute with methanol to volume,and mix.
Amphotericin Bstandard preparation—
Dissolve about 50mg of USP Amphotericin B RS,accurately weighed,in 10.0mLof dimethyl sulfoxide in a 50-mLvolumetric flask,dilute with methanol to volume,and mix.Transfer 4.0mLof this solution to a 50-mLvolumetric flask,dilute with methanol to volume,and mix.Prepare this solution fresh daily.
Procedure—
Concomitantly determine the absorbances of the Nystatinand Amphotericin Bstandard preparationsand the Test preparationin 1-cm cells at 304nm and at 282nm,with a suitable spectrophotometer,using a 1in 62.5solution of dimethyl sulfoxide in methanol as the blank.Calculate the percentage of amphotericin Ataken by the formula:
in which WNis the weight,in mg,of USP Nystatin RStaken,AB282and AB304are the absorbances of the Amphotericin Bstandard preparationat 282nm and 304nm,respectively,AN282andAN304are the absorbances of the Nystatin standard preparationat 282nm and 304nm,respectively,AU282and AU304are the absorbances of the Test preparationat 282nm and 304nm,respectively,and WUis the weight,in mg,of the Amphotericin Btaken:not more than 5%,calculated on the dried basis,is found.[NOTE—Amphotericin Bintended for use in preparing dermatological creams,lotions,and ointments,and oral suspensions and capsules,contains not more than 15%of amphotericin A,calculated on the dried basis.]
Assay—
Proceed with amphotericin Bas directed under Antibiotics—Microbial Assays á81ñ.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 150
Phone Number:1-301-816-8335