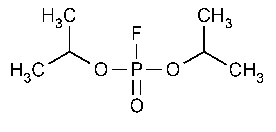
Isoflurophate
Phosphorofluoridic acid,bis(1-methylethyl)ester.
Diisopropyl phosphorofluoridate [55-91-4].
»Isoflurophate contains not less than 95.0percent of C6H14FO3P.
Caution—Handle Isoflurophate with exceptional care since it is very toxic.Wear full-face breathing apparatus and gloves.Open the container only in a hood.
Packaging and storage—
Preserve in glass,fuse-sealed containers,or in other suitable sealed containers,in a cool place.
Labeling—
Label it to indicate that in the handling of Isoflurophate in open containers,the eyes,nose,and mouth are to be protected with a suitable mask,and contact with the skin is to be avoided.
Identification—
A:
Under a hood with a good draftplace a few drops of Isoflurophate in a small platinum crucible,quickly add 2mLof sulfuric acid,and immediately cover the crucible with a small,clear watch glass.Allow to stand for 10minutes,then heat on a steam bath for 5to 10minutes:the side of the watch glass exposed to the mixture is visibly etched.
B:
Slowly heat under the hoodthe crucible and contents from Identificationtest Auntil copious white fumes are evolved.Cool,place the crucible in a beaker,and add a sufficient quantity of water nearly to cover the crucible.After a few minutes remove the crucible from the beaker with the aid of a glass rod,add 3mLof nitric acid,and boil for a few minutes.Cool,cautiously add 6Nammonium hydroxide with stirring until a slight odor of ammonia persists,then add 1mLof nitric acid.Filter the liquid if not clear,warm to about 40 ,and add about 10mLof ammonium molybdate TS:a yellow precipitate,which is soluble in 6Nammonium hydroxide,is formed.
,and add about 10mLof ammonium molybdate TS:a yellow precipitate,which is soluble in 6Nammonium hydroxide,is formed.
Acidity—
Mixed indicator—
Mix 3volumes of a 1in 1000solution of bromocresol green in alcohol and 1volume of a 1in 500solution of methyl red in alcohol.
0.1N Sodium hydroxide in dehydrated alcohol—
Dissolve about 0.4g of sodium hydroxide in 100mLof dehydrated alcohol,and when solution is complete,standardize the alcoholic solution as follows.Pipet 25.0mLof 0.1Nhydrochloric acid VSinto a suitable container.Dilute with 50mLof water,add 2drops of Mixed indicator,and titrate with the alcoholic sodium hydroxide solution to the first appearance of a green color.Calculate the normality.[NOTE—Store in tightly stoppered bottles.]
Test preparation—
Prepare as directed in the Assay,except to draw 1.0mLinstead of 0.5mLof Isoflurophate into the bulb.
Procedure—
Carefully place the bulb containing the Test preparationin a 250-mLflask containing 20mLof dehydrated alcohol.Break the bulb as directed in the Procedureunder Ionic fluorine.Rinse the glass tube with 30mLof dehydrated alcohol,and titrate immediately with 0.1N Sodium hydroxide in dehydrated alcohol,using the Mixed indicator,to the appearance of the first green color.Each mLof 0.1N Sodium hydroxide in dehydrated alcoholis equivalent to 100.8µg of hydrogen ion (acidity).The limit is 0.01%.
Ionic fluorine—
Sodium methoxide solution—
Dissolve 10g of sodium methoxide in dehydrated alcohol to make 500mL,and mix.
Buffer solution—
Dissolve 9.55g of monochloroacetic acid and 2g of sodium hydroxide in water to make 100mL.If necessary,adjust by the addition,of either reagent to obtain a solution having a pHof 3.0.
Standard fluoride solution—
Dissolve 2.2105g of sodium fluoride in water to make 1000.0mL.Each mLis equivalent to 1.00mg of fluoride ion.
Thorium nitrate solution—
Dissolve 9g of thorium nitrate in water to make 1000.0mL,and mix.Standardize as directed under Standard curve.
Standard curve—
Into each of four 180-mLbeakers pipet 50.0mLof Sodium methoxide solutionand 0.25,0.50,1.0,and 2.0mL,respectively,of Standard fluoride solution.Treat each beaker in the same manner,as follows.Add 2drops of phenolphthalein TS,and render just acid with 6Nhydrochloric acid.Add 1.0mLof sodium alizarinsulfonate solution (1in 2000),and add 6Nhydrochloric acid dropwise until the pink color is discharged.Dilute with water to 100mL,and add Buffer solution(approximately 4mL)until the pHis 3.1.Titrate with Thorium nitrate solution,while stirring constantly and rapidly,to a permanent pink color.During the titration,maintain the solution at a pHbetween 2.9and 3.1by adding small volumes of Buffer solution,if necessary,but not more than a total of 10mL.Plot the mg of fluoride ion versus the mLof Thorium nitrate solutionconsumed.
Test preparation—
Prepare as directed in the Assay,except to draw 1.0mLinstead of 0.5mLof Isoflurophate into the bulb.
Procedure—
Place 50.0mLof Sodium methoxide solutionin a 180-mLbeaker,and add 2drops of phenolphthalein TS.Acidify,dropwise,with 6Nhydrochloric acid.Add 1.0mLof sodium alizarinsulfonate solution (1in 2000),then add 6Nhydrochloric acid dropwise until the pink color disappears.Add 0.5Nsodium hydroxide until a faint pink color appears,then add 0.05Nhydrochloric acid until the pink color just disappears.Add 4mLof the Buffer solution.Carefully place the Test preparationin the beaker with the stem of the bulb inserted in a suitable length of glass tubing.Break the bulb by pressing down on the glass tubing,making sure that the bulb is beneath the surface of the liquid and that the bottom of the beaker is properly supported so that it will not break when the bulb is broken.Wash down the glass tube with water,and dilute with water to 100mL.Titrate immediately with the Thorium nitrate solution.Determine the mg of ionic fluorine present in the Test preparationdirectly from the thorium nitrate standardization curve.Not more than 0.15%of ionic fluorine is found.
Assay—
Solvent—
Use dry carbon disulfide,chromatographic grade.
Internal standard solution—
Pipet 1.0mLof chromatographic grade cyclohexanone into a 100-mLvolumetric flask,dilute with Solventto volume,and mix.Pipet 3.0mLof the resulting solution into a 100-mLvolumetric flask,dilute with Solventto volume,and mix.Each mLof the Internal standard solutioncontains 0.30µLof cyclohexanone.
Standard preparation—
Dissolve a suitable quantity of Isoflurophate,previously subjected to the Assay,in peanut oil,and dilute quantitatively and stepwise with peanut oil to obtain a solution having a known concentration of about 0.8mg of isoflurophate per g of solution.Transfer about 1.2g of this Isoflurophate solution in peanut oil,accurately weighed,to a 10-mLvolumetric flask,pipet 1.0mLof Internal standard solutioninto the flask,dilute with Solventto volume,and mix.
Assay preparation—
Tare an unsealed,thin-walled glass bulb with a thin,long stem,having a capacity of 1to 2mL.Under a hood,open the Isoflurophate container and place it in a firmly based container in a suitable vacuum-filtration flask.Insert the stem of the bulb under the surface of the liquid,and insert the stopper in the filtration flask.Allow about 0.5mLof liquid to be drawn up into the bulb,and release the vacuum.Remove the bulb from the container,wipe the stem clean,fire-seal it without loss of any glass,cool,and again weigh.
Place the glass bulb in a 125-mLconical flask,add about 70mLof Solvent,and break the bulb with the aid of a glass rod by pressing down on the glass tubing over the neck of the bulb.Take care to assure that the bulb is beneath the surface of the liquid in the flask and that the bottom of the flask is properly supported so that it will not break when the bulb is broken.Remove the rod,transfer the solution to a 100-mLvolumetric flask,dilute with Solventto volume,and mix (Solution A).Pipet 2.0mLof Solution Ainto a 10-mLvolumetric flask,dilute with Solventto volume,and mix (Solution B).Pipet 1.0mLof Solution Binto another 10-mLvolumetric flask,pipet 1.0mLof Internal standard solutioninto the flask,dilute with Solventto volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—Under typical conditions,the gas chromatograph is equipped with a flame-ionization detector,and contains a 1.8-m ×4-mm glass column packed with 5%phase G33on 80-to 100-mesh support S1AB,utilizing either a glass-lined sample introduction system or on-column injection.The column is maintained isothermally at a temperature between 75 and 80
and 80 ,and the injector port and detector block are maintained at 200
,and the injector port and detector block are maintained at 200 and 250
and 250 ,respectively;dry,oxygen-free helium is used as the carrier gas at a flow rate adjusted to obtain a cyclohexanone peak about 6minutes after sample introduction.
,respectively;dry,oxygen-free helium is used as the carrier gas at a flow rate adjusted to obtain a cyclohexanone peak about 6minutes after sample introduction.
Procedure—
Inject 6µLof the Standard preparationinto a suitable gas chromatograph,and record the chromatogram.Measure the areas under the first (cyclohexanone)and second (isoflurophate)peaks,and record the values as ADand AS,respectively.Calculate the factor Ftaken by the formula:
(AD/AS)(WS/10)(C/1000),
in which WSis the weight,in mg,of Isoflurophate solution in peanut oil in the Standard preparation,and Cis the weight,in mg,of isoflurophate per g of Isoflurophate solution in peanut oil.Similarly inject 6µLof the Assay preparation,and record the chromatogram.Measure the areas under the first (cyclohexanone)and second (isoflurophate)peaks,and record the values as aDand aU,respectively.Calculate the percentage of C6H14FO3Pin the portion of Isoflurophate taken by the formula:
(F)(aU/aD)(100/WU)(5000),
in which WUis the weight,in mg,of Isoflurophate in the Assay preparation,and Fis the factor as determined above.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 1071
Phone Number:1-301-816-8330