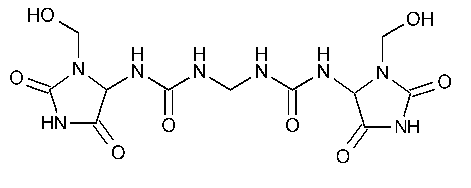
Imidurea
N,N¢¢-Methylenebis[N¢-[3-(hydroxymethyl)-2,5-dioxo-4-imidazolidinyl]urea].
1,1¢-Methylenebis[3-[3-(hydroxymethyl)-2,5-dioxo-4-imidazolidinyl]urea]. [39236-46-9].
»Imidurea contains not less than 26.0percent and not more than 28.0percent of nitrogen (N),calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Color and clarity of solution—
Dissolve 3.0g in 7.0mLof water in a test tube:the solution is clear and colorless.
Identification,Infrared Absorption á197Kñ.
pHá791ñ:
between 6.0and 7.5,in a solution (1in 100).
Loss on drying á731ñ—
Dry in vacuum over phosphorus pentoxide for 48hours:it loses not more than 3.0%of its weight.
Residue on ignition á281ñ:
not more than 3.0%.
Heavy metals,Method IIá231ñ:
0.001%.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Nitrogen content—
Place about 150mg of Imidurea,accurately weighed,in a 500-mL Kjeldahl flask,and add 8.0g of anhydrous sodium sulfate,0.5g of anhydrous cupric sulfate,0.1g of yellow mercuric oxide,and 11mLof sulfuric acid.Incline the flask at an angle of about 45 ,and gently heat the mixture,keeping the temperature below the boiling point until frothing has ceased.Increase the heat until the acid boils briskly,and continue the heating until the solution has become clear green in color or practically colorless for 30minutes.Allow to cool,cautiously add 225mLof water,mix the contents of the flask,and again cool.Add cautiously 1.0g of zinc metal dust,2.0g of sodium thiosulfate,and 18.0g of sodium hydroxide pellets,and without delay connect the flask to a Kjeldahl connecting bulb (trap),previously attached to a condenser,the delivery tube of which dips beneath the surface of 50mLof boric acid solution (1in 25)contained in a 300-mLconical flask.Mix the contents of the Kjeldahl flask by gentle rotation,and distill about 125mLinto the receiver.Add not less than 3drops of methyl red-methylene blue TSto the contents of the receiving vessel,and determine the ammonia by titration with 0.1Nhydrochloric acid VS.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nhydrochloric acid is equivalent to 1.401mg of nitrogen.
,and gently heat the mixture,keeping the temperature below the boiling point until frothing has ceased.Increase the heat until the acid boils briskly,and continue the heating until the solution has become clear green in color or practically colorless for 30minutes.Allow to cool,cautiously add 225mLof water,mix the contents of the flask,and again cool.Add cautiously 1.0g of zinc metal dust,2.0g of sodium thiosulfate,and 18.0g of sodium hydroxide pellets,and without delay connect the flask to a Kjeldahl connecting bulb (trap),previously attached to a condenser,the delivery tube of which dips beneath the surface of 50mLof boric acid solution (1in 25)contained in a 300-mLconical flask.Mix the contents of the Kjeldahl flask by gentle rotation,and distill about 125mLinto the receiver.Add not less than 3drops of methyl red-methylene blue TSto the contents of the receiving vessel,and determine the ammonia by titration with 0.1Nhydrochloric acid VS.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nhydrochloric acid is equivalent to 1.401mg of nitrogen.
Auxiliary Information—
Staff Liaison:Catherine Sheehan,B.Sc.,Scientist
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 3021
Phone Number:1-301-816-8262