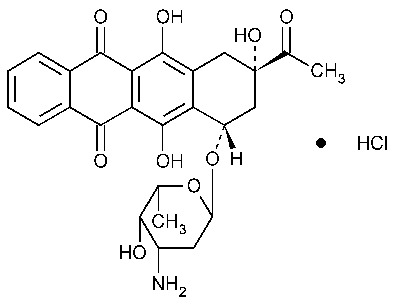
Idarubicin Hydrochloride
5,12-Naphthacenedione,9-acetyl-7-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,9,11-trihydroxyhydrochloride,(7S-cis)-.
(1S,3S)-3-Acetyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-6,11-dioxo-1-naphthacenyl 3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside,hydrochloride [57852-57-0].
»Idarubicin Hydrochloride contains not less than 960µg and not more than 1030µg of C26H27NO9·HCl per mg,calculated on the anhydrous basis.
Caution—Great care should be taken to prevent inhaling particles of Idarubicin Hydrochloride and exposing the skin to it.
Packaging and storage—
Preserve in tight containers.
Labeling—
The amorphous form is so labeled.
Identification—
Crystallinity á695ñ:
meets the requirements,except where it is labeled as amorphous,most of the particles do not exhibit birefringence and extinction positions.
pHá791ñ:
between 5.0and 6.5,in a solution containing 5mg per mL.
Water,Method Iá921ñ:
not more than 5.0%.
Chromatographic purity—
Using the chromatogram of theAssay preparationobtained in theAssay,and disregarding the solvent peak,calculate the percentage of each impurity taken by the formula:
100rI/rS,
in whichriis the response of each impurity peak;andrSis the sum of the responses of all the peaks:not more than 1.0%of any individual impurity is found;and the sum of all impurities is not more than 3.0%.
Assay—
Mobile phase—
Prepare a mixture of water,acetonitrile,methanol,and phosphoric acid (540:290:170:2).Dissolve 1g of sodium lauryl sulfate in 1000mLof this solution,adjust with 2Nsodium hydroxide to a pHof 3.6±0.1,pass through a filter having a porosity of 0.5µm or finer,and degas.Make adjustments if necessary (seeSystem SuitabilityunderChromatography á621ñ).
Diluent—
Prepare as directed forMobile phase,except to omit the sodium lauryl sulfate.
Standard preparation—
Dissolve an accurately weighed quantity of USP Idarubicin Hydrochloride RSinDiluentto obtain a solution having a known concentration of about 500µg of idarubicin hydrochloride per mL.
Assay preparation—
Transfer about 50mg of Idarubicin Hydrochloride,accurately weighed,to a 100-mLvolumetric flask,dissolve inDiluent,dilute withDiluentto volume,and mix.
Resolution solution—
Prepare an aqueous solution containing 1mg of Idarubicin Hydrochloride per mL.To 2mLof this solution in a test tube,add 20µLof hydrochloric acid,and heat in an oil bath at 95 for about 8minutes.Mix 1mLof this solution and 9mLofDiluent.ThisResolution solutioncontains a mixture of 4-demethoxydaunorubicinone and idarubicin.
for about 8minutes.Mix 1mLof this solution and 9mLofDiluent.ThisResolution solutioncontains a mixture of 4-demethoxydaunorubicinone and idarubicin.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column that contains packing L13.The flow rate is about 2mLper minute.Chromatograph theResolution solution,and record the peak responses as directed forProcedure:the relative retention times are about 0.5for 4-demethoxydaunorubicinone and 1.0for idarubicin;and the resolution,R,between the 4-demethoxydaunorubicinone peak and the idarubicin peak is not less than 9.5.Chromatograph theStandard preparation,and record the peak responses as directed forProcedure:the capacity factor,k¢,for the idarubicin peak is not less than 10and not more than 20;the tailing factor for the idarubicin peak is not less than 0.85and not more than 1.2;the column efficiency calculated from the idarubicin peak is not less than 3000theoretical plates;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of theStandard preparationand theAssay preparationinto the chromatograph,record the chromatograms,and measure the areas for the major peaks.Calculate the quantity,in µg,of C26H27NO9·HCl in each mg of the Idarubicin Hydrochloride taken by the formula:
100(C/M)(rU/rS),
in whichCis the concentration,in µg per mL,of idarubicin hydrochloride (C26H27NO9·HCl)in theStandard preparation;Mis the quantity,in mg,of Idarubicin Hydrochloride taken to prepare theAssay preparation;andrUandrSare the responses of the idarubicin peak obtained from theAssay preparationand theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 995
Pharmacopeial Forum:Volume No.27(6)Page 3302
Phone Number:1-301-816-8335