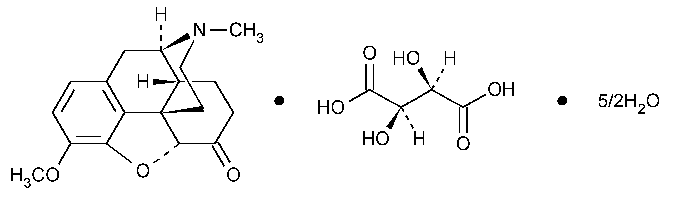
Hydrocodone Bitartrate
C18H21NO3·C4H6O6·2½H2O
494.490
Morphinan-6-one,4,5-epoxy-3-methoxy-17-methyl-,(5a)-,[R-(R*,R*)]-2,3-dihydroxybutanedioate (1:1),hydrate (2:5).
4,5a-Epoxy-3-methoxy-17-methylmorphinan-6-one tartrate (1:1)hydrate (2:5) [34195-34-1;6190-38-1].
Morphinan-6-one,4,5-epoxy-3-methoxy-17-methyl-,(5a)-,[R-(R*,R*)]-2,3-dihydroxybutanedioate (1:1),hydrate (2:5).
4,5a-Epoxy-3-methoxy-17-methylmorphinan-6-one tartrate (1:1)hydrate (2:5) [34195-34-1;6190-38-1].
Anhydrous 449.46 [143-71-5].
»Hydrocodone Bitartrate,dried in vacuum at 105 for 2hours,contains not less than 98.0percent and not more than 102.0percent of C18H21NO3·C4H6O6.
for 2hours,contains not less than 98.0percent and not more than 102.0percent of C18H21NO3·C4H6O6.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
A:Infrared Absorption á197Mñ.
B:Ultraviolet Absorption á197Uñ—
Solution:
100µg per mL.
Medium:
0.1Nsulfuric acid.
Specific rotation á781Sñ:
between –79 and –84
and –84 .
.
Test solution:
20mg,undried,per mL,in water.Calculate the result on the basis of the undried aliquot.
pHá791ñ:
between 3.2and 3.8,in a solution (1in 50).
Loss on drying—
Dry it in vacuum at 105 for 2hours [NOTE—See the Notein the Assayfor precautions regarding handling of the dried material.]:it loses not less than 7.5%and not more than 12.0%of its weight.
for 2hours [NOTE—See the Notein the Assayfor precautions regarding handling of the dried material.]:it loses not less than 7.5%and not more than 12.0%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Chloride—
To 10mLof a solution (1in 100),acidified with nitric acid,add a few drops of silver nitrate TS:no opalescence is produced immediately.
Delete the following:
Test solution:
a mixture of methanol and water (1:1).
Standard solution:
a mixture of methanol and water (1:1).
Eluant:
a mixture of hexanes,acetone,methanol,and ammonium hydroxide (60:40:20:1.5).
Visualization:
3,followed by overspraying with hydrogen peroxide TS.[NOTE—Cover the thin-layer chromatographic plate with a glass plate to slow fading of the spots.Exclude the origin spot,if present,from the determination of the total impurities.] USP28
USP28
Add the following:
Solution A,Solution B,Mobile phase,and Chromatographic system—
Proceed as directed in theAssay.
Test solution—
Use theAssay preparation.
System suitability solution—
Combine about 1.5mg of USP Dihydrocodeine Bitartrate RSand 1.0mLof theStandard preparation prepared for theAssay in a 200-mLvolumetric flask.Dilute withSolution Ato volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
Proceed as directed in theAssay.Chromatograph theSystem suitability solution,and record the peak responses as directed forProcedure:the relative retention times are about 0.89for dihydrocodeine and 1.0for hydrocodone;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Inject equal volumes (about 20µL)of theTest solution and theSystem suitability solution into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentage of dihydrocodeine bitartrate and any unknown impurities in the portion of Hydrocodone Bitartrate taken by the formula:
 USP28
USP28
10,000(C/W)F(rU/rS),
in whichCis the concentration,in mg per mL,of USP Hydrocodone Bitartrate RSin theSystem suitability solution;Wis the quantity,in mg,of Hydrocodone Bitartrate taken to prepare theTest solution;Fis the relative response factor and is equal to the values given in the following table;rUis the individual peak response of each impurity in the test solution;andrSis the response of hydrocodone bitartrate in theSystem suitability solution:not more than 0.5%of any individual impurity is found,and not more than 2.0%of total impurities is found.
| Hydrocodone Bitartrate and Related Compounds |
Relative Retention Time |
Relative Response Factor |
| dihydrocodeine bitartrate | 0.89 | 0.81 |
| hydrocodone diol bitartrate |
0.92 | 0.76 |
| hydrocodone bitartrate | 1.00 | 1.00 |
| dihydrothebainone bitartrate |
1.03 | — |
| hydrocodone aldol dimer bitartrate |
1.10 | 0.96 |
| 7-cyclohexenyl hydroco- done bitartrate |
1.50 | 1.00 |
| benzophenone | 1.79 | — |
| other impurities | — | 1.0 |
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Change to read:
Assay—
[
Solution A—
Dissolve 5.75g of monobasic ammonium phosphate in about 900mLof water in a 1000-mLvolumetric flask,adjust with phosphoric acid to a pHof 3.0±0.1,dilute with methanol to volume,and mix.
Solution B—
Prepare a filtered and degassed mixture of methanol and water (80:20).
Mobile phase—
Use variable mixtures ofSolution AandSolution Bas directed forChromatographic system.Make adjustments if necessary (seeSystem Suitability underChromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Hydrocodone Bitartrate RSinSolution Ato obtain a solution having a known concentration of about 1.5mg per mL.
Assay preparation—
Transfer an accurately weighed quantity of previously dried Hydrocodone Bitartrate,equivalent to about 150mg of hydrocodone bitartrate,to a 100-mLvolumetric flask,dissolve in and dilute withSolution Ato volume,and mix.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 280-nm detector and a 4.6-mm ×25-cm column that contains 5-µm packing L7.The column temperature is maintained at 60 .The flow rate is about 1.2mLper minute.The chromatograph is programmed as follows.
.The flow rate is about 1.2mLper minute.The chromatograph is programmed as follows.
Chromatograph theStandard preparation,and record the peak responses as directed forProcedure:the tailing factor is not more than 1.0;and the relative standard deviation for replicate injections is not more than 2.0%.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 100 | 0 | equilibrium |
| 0–6 | 100 | 0 | isocratic |
| 6–30 | 100®0 | 0®100 | linear gradient |
| 30–31 | 0®100 | 100®0 | linear gradient |
Procedure—
Separately inject equal volumes (about 20µL)of theStandard preparation and theAssay preparation into the chromatograph,record the chromatograms,and measure the peak responses.Calculate the quantity,in mg,of hydrocodone bitartrate (C18H21NO3·C4H6O6)in the portion of Hydrocodone Bitartrate taken by the formula:
 USP28
USP28
100C(rU/rS),
in whichCis the concentration,in mg per mL,of USP Hydrocodone Bitartrate RSin theStandard preparation;andrUandrSare the peak responses obtained from theAssay preparation and theStandard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 956
Pharmacopeial Forum:Volume No.30(5)Page 1628
Phone Number:1-301-816-8139