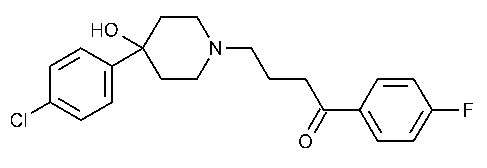
Haloperidol
1-Butanone,4-[4-(4-chlorophenyl)-4-hydroxy-1-piperidinyl]-1-(4-fluorophenyl)-.
4-[4-(p-Chlorophenyl)-4-hydroxypiperidino]-4¢-fluorobutyrophenone [52-86-8].
»Haloperidol contains not less than 98.0percent and not more than 102.0percent of C21H23ClFNO2,calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
B:Ultraviolet Absorption á197Uñ—
Solution:
20µg per mL.
Medium:
dilute hydrochloric acid (1in 100)in isopropyl alcohol (1in 9).
Absorptivities at 245nm,calculated on the dried basis,do not differ by more than 3.0%.
Loss on drying á731ñ—
Dry it in vacuum at 60 for 3hours:it loses not more than 0.5%of its weight.
for 3hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Limit of haloperidol related compound A—
Test solution—
Dissolve about 80mg of Haloperidol,accurately weighed,in 80mLof isopropyl alcohol in a 100-mLvolumetric flask.Add 10mLof dilute hydrochloric acid (1in 100),dilute with isopropyl alcohol to volume,and mix.
Standard solution—
Prepare a solution containing 800µg per mLof USP Haloperidol RSand 8µg per mLof USP Haloperidol Related Compound A RSin isopropyl alcohol containing 10mLof dilute hydrochloric acid (1in 100)in each 100mLof solution.
Procedure—
Concomitantly determine the absorbances of theTest solutionand theStandard solutionat the wavelength of maximum absorbance at about 335nm,with a suitable spectrophotometer,using isopropyl alcohol containing 10mLof dilute hydrochloric acid (1in 100)in each 100mLof solution as the blank.The absorbance of theTest solutionis not greater than that of theStandard solution,corresponding to not more than 1.0%.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay—
Dissolve about 125mg of Haloperidol,accurately weighed,in 25mLof glacial acetic acid,add 3drops ofp-naphtholbenzein TS,and titrate with 0.05Nperchloric acid VS.Perform a blank determination,and make any necessary correction.Each mLof 0.05Nperchloric acid is equivalent to 18.79mg of C21H23ClFNO2.
Auxiliary Information—
Staff Liaison:Ravi Ravichandran,Ph.D.,Senior Scientist
Expert Committee:(PA3)Pharmaceutical Analysis 3
USP28–NF23Page 937
Pharmacopeial Forum:Volume No.29(6)Page 1897
Phone Number:1-301-816-8330