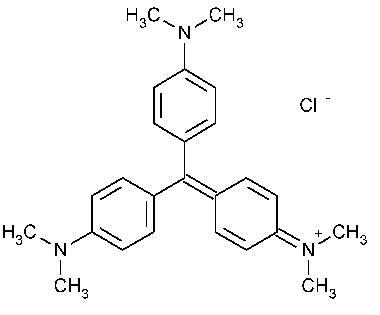
Gentian Violet
Methanaminium,N-[4-[bis[4-(dimethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]-N-methyl-,chloride.
C.I.Basic violet 3.
[4-[Bis[p-(dimethylamino)phenyl]methylene]-2,5-cyclohexadien-1-ylidene]dimethylammonium chloride [548-62-9].
»Gentian Violet contains not less than 96.0percent and not more than 100.5percent of gentian violet (C25H30ClN3),calculated on the anhydrous basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
Sprinkle about 1mg on 1mLof sulfuric acid:it dissolves in the acid with an orange or brown-red color.When this solution is diluted cautiously with water,the color changes to brown,then to green,and finally to blue.
B:
Dissolve about 20mg in 10mLof water,and add 5drops of hydrochloric acid.To 5mLof this solution add tannic acid TSdropwise:a deep blue precipitate is formed.
C:
To the remainder of the solution prepared for Identificationtest Badd about 500mg of zinc dust,and warm the mixture:rapid decolorization occurs.Place a drop of the decolorized solution adjacent to a drop of 6Nammonium hydroxide on a filter paper:a blue color is produced at the zone of contact.
Water,Method Iá921ñ:
not more than 7.5%.
Residue on ignition á281ñ:
not more than 1.5%.
Alcohol-insoluble substances—
Boil 1.0g,accurately weighed,with 50mLof alcohol under a reflux condenser for 15minutes,filter through a tared filtering crucible,wash the residue on the filter with hot alcohol until the last washing is not colored violet,and dry the crucible at 105 for 1hour:not more than 1.0%of insoluble residue remains.
for 1hour:not more than 1.0%of insoluble residue remains.
Arsenic,Method Iá211ñ—
Mix 300mg with about 2.5g each of powdered potassium nitrate and anhydrous sodium carbonate,and heat the mixture in a crucible until the organic matter is completely oxidized.Dissolve the cooled residue in 15mLof 2Nsulfuric acid,and evaporate the solution by heating until copious white fumes begin to evolve.Dissolve the residue in 35mLof water.The limit is 0.001%.
Lead—
Place 1.0g in a small Kjeldahl flask,add 5mLof sulfuric acid,and insert a small funnel into the flask.Gently rotate the flask until the sulfuric acid has completely wetted the Gentian Violet,then heat gently until complete carbonization has taken place.Allow to cool,and add,in small quantities,5mLof nitric acid.Again heat gently until copious white fumes are evolved.Allow to cool,add another 5mLof nitric acid,and again heat until white fumes are evolved.Allow to cool,cautiously add about 25mLof water,and boil for a few minutes.After cooling,neutralize to litmus paper with ammonium hydroxide,and add 5mLof nitric acid.Transfer the solution to a 100-mLvolumetric flask,dilute with water to volume,and mix.Use 20mLof this solution for the limit test for Lead á251ñ.Perform a blank determination,and make any necessary correction.The limit is 0.003%.
Zinc—
Zinc standard stock solution—
Transfer about 1g of zinc,accurately weighed,to a 1000-mLvolumetric flask,add 50mLof nitric acid,and mix to dissolve.Dilute with water to volume,and mix.
Standard preparation—
Dilute the Zinc standard stock solutionwith water to obtain a Standard preparationcontaining 0.50µg of zinc per mL.
Test preparation—
Weigh accurately 0.50g of Gentian Violet in a suitable tared crucible.Place in a low-temperature plasma ashing apparatus,and ash until a constant weight is attained.Pipet 10mLof 6Nnitric acid into the crucible,and heat to dissolve the ash.Transfer the solution to a 500-mLvolumetric flask,dilute with water to volume,and mix.Prepare a reagent blank.
Procedure—
Concomitantly determine the absorbances of the Standard preparation,the Test preparation,and the reagent blank at the zinc emission line at 213.9nm,with a suitable atomic absorption spectrophotometer (see Spectrophotometry and Light-scattering á851ñ)equipped with a zinc lamp and an air–acetylene flame,using water as the blank.The absorbance of the Test preparation,corrected for that of the reagent blank,is not greater than the absorbance of the Standard preparation,similarly corrected (0.05%).
Chromatographic purity—
Dissolve 10mg in 10mLof methanol to obtain the Test solution.Transfer 1.0mLof Test solutionto a 100-mLvolumetric flask,dilute with methanol to volume,and mix (Diluted test solution).Apply 5µLeach of the Test solutionand the Diluted test solutionto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of octadecylsilanized chromatographic silica gel.Allow the spots to dry,and develop the chromatogram in a suitable chromatographic chamber with a solvent system consisting of the upper layer separated from a well-shaken mixture of water,butyl alcohol,and glacial acetic acid (100:80:20),until the solvent front has moved about three-fourths of the length of the plate.Remove the plate from the chamber,allow the solvent to evaporate,and visually locate the spots on the plate:the Test solutionexhibits a principal spot and not more than one secondary spot which,if present in the chromatogram from the Test solution,is not more intense than the principal spot obtained from the Diluted test solution(1.0%).
Assay—
Transfer about 400mg of Gentian Violet,accurately weighed,to a 300-mLconical flask,add 25mLof water and 10mLof hydrochloric acid,displace the air in the flask with carbon dioxide,and pass a stream of carbon dioxide through the flask during the assay.Add 50.0mLof 0.1Ntitanium trichloride VS,heat to boiling,and boil gently for 10minutes,swirling the liquid occasionally.Cool the solution,add 5mLof ammonium thiocyanate solution (1in 10),and titrate with 0.1Nferric ammonium sulfate VSuntil a faint red color is produced.Perform a blank determination (see Residual Titrations á541ñ).Each mLof 0.1Ntitanium trichloride is equivalent to 20.40mg of C25H30ClN3.
Auxiliary Information—
Staff Liaison:Behnam Davani,Ph.D.,MBA,Senior Scientist
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 901
Phone Number:1-301-816-8394