Felodipine Extended-Release Tablets
»Felodipine Extended-Release Tablets contain not less than 90.0percent and not more than 110.0percent of the labeled amount of felodipine (C18H19Cl2NO4).
Packaging and storage—
Preserve in tight containers.
Identification—
The retention time of the major peak in the chromatogram of the Assay preparationcorresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
Change to read:
Drug release á724ñ—
Apparatus 2:
50rpm.
Times:
2,6,and 10hours.
Buffer solution—
Prepare as directed in the Assay.
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solution,acetonitrile,and methanol (2:2.5:1).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard stock solution—
Dissolve an accurately weighed quantity of USP Felodipine RSin alcohol to obtain a solution having a known concentration of 0.25mg per mL.
Standard solution—
Dilute an accurately measured volume of the Standard stock solutionquantitatively,and stepwise if necessary,with Mediumto obtain a solution having a known concentration of USP Felodipine RSequivalent to the concentration that would result from about 60%dissolution of a single Tablet in 500mLof Medium.
Test solution—
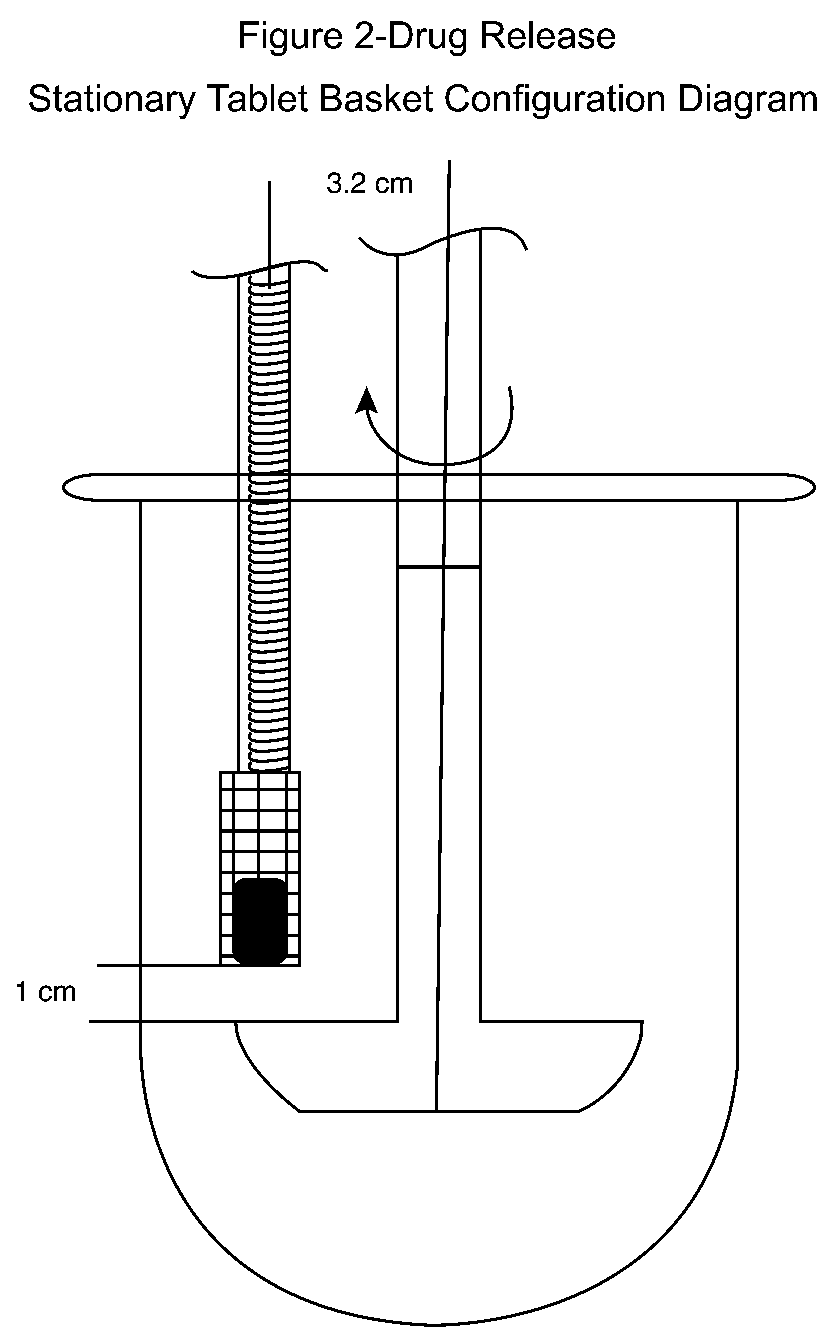
Place each Tablet in a specially made quadrangular basket of stainless steel wire gauze,soldered in one of its upper,narrow sides to the end of a steel rod (see Figure 1).Place the tablet cover in the horizontal diagonal of the basket.Put the rod assembly up through the cover of the dissolution vessel,and fix it by means of two teflon nuts,3.2cm from the center of the vessel,or by any other appropriate means.Adjust the lower edge of the bottom of the basket to approximately 1cm above the top of the paddle blade (see Figure 2).Orient the large side of the basket tangentially to the flow stream with the Tablet standing on its edge.Pass a 10-mLportion of the solution under test,obtained at each time interval,through a suitable filter.
Chromatographic system—
Proceed as directed in the Assay.
Procedure—
Separately inject equal volumes (100µL)of the Standard solutionand the Test solution into the chromatograph,record the chromatograms,and measure the areas for the major peaks.Calculate the quantity,in mg,of felodipine (C18H19Cl2NO4)dissolved in the Mediumby the formula:
 USP28
USP28
CD(rU/rS),
in which Cis the concentration,in mg per mL,of USP Felodipine RSin the Standard solution;Dis the dilution factor used in preparing the Test solution;and rUand rSare the felodipine peak areas obtained from the Test solutionand the Standard solution,respectively.
Tolerances—
The percentages of the labeled amount of C18H19ClNO4dissolved at the times specified conform to Acceptance Table 1 under Drug Release á724ñ.
under Drug Release á724ñ. USP28
USP28
| Time (hours) | Amount dissolved |
| 2 | between 10%and 30% |
| 6 | between 42%and 68% |
| 10 | not less than 75% |
Uniformity of dosage units á905ñ:
meet the requirements.
PROCEDURE FOR CONTENT UNIFORMITY—
Buffer solution,Mobile phase,Standard stock preparation,and Chromatographic system—
Proceed as directed in the Assay.
Standard solution—
Use the Standard preparation,prepared as directed in the Assay.
Test solution—
Transfer 1Tablet to a 100-mLvolumetric flask,add 40mLof acetonitrile,and sonicate for 20minutes with occasional swirling.Add 20mLof methanol,and shake by mechanical means for 30minutes.Allow to cool to room temperature,dilute with Buffer solutionto volume,and mix.Centrifuge a portion of the solution at high speed for 15minutes.Dilute a portion of the supernatant withMobile phaseto obtain a solution containing about 20µg of felodipine per mL.Pass this solution through a filter having a 0.5-µm or finer porosity.
Procedure—
Separately inject equal volumes (about 40µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of felodipine (C18H19Cl2NO4)in the Tablet taken by the formula:
(TC/D)(rU/rS),
in which Tis the labeled quantity,in mg,of felodipine in the Tablet;Cis the concentration,in µg per mL,of USP Felodipine RSin the Standard solution;Dis the concentration,in µg per mL,of felodipine in the Test solution,based on the labeled quantity per Tablet and the extent of dilution;and rUand rSare the felodipine peak responses obtained from the Test solutionand the Standard solution,respectively.
Related compounds—
Buffer solution and Mobile phase—
Prepare as directed in the Assay.
Standard stock solution 1—
Dissolve an accurately weighed quantity of USP Felodipine Related Compound A RSin methanol to obtain a solution having a known concentration of about 0.2mg per mL.Transfer 10.0mLof this solution to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Standard stock solution 2—
Dissolve an accurately weighed quantity of USP Felodipine RSin methanol,and dilute quantitatively,and stepwise if necessary,with methanol to obtain a solution having a known concentration of about 2mg per mL.
System suitability solution—
Transfer 15.0mLof Standard stock solution 1and 5.0mLof Standard stock solution 2to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Standard solution—
Transfer 10.0mLof Standard stock solution 1to a 100-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.
Test solution—
Proceed as directed for the Assay preparationin the Assay,except that after centrifuging a portion of the solution at high speed for 15minutes,pass a portion of the supernatant through a filter having a 0.5-µm or finer porosity,discarding the first 4mLof the filtrate.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×15-cm column that contains packing L1.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are 0.75for felodipine related compound Aand 1.0for felodipine;the resolution,R,between felodipine and felodipine related compound Ais not less than 1.5;and the column efficiency is not less than 1500theoretical plates.
Procedure—
Separately inject equal volumes (about 40µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the peak responses for felodipine related compound A.Calculate the percentage of felodipine related compound Ain the portion of Tablets taken by the formula:
1000C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Felodipine Related Compound A RSin the Standard solution;and rUand rSare the peak responses of felodipine related compound Aobtained from the Test solutionand the Standard solution,respectively:not more than 2.0%is found.
Assay—
Buffer solution—
Dissolve 6.9g of monobasic sodium phosphate in about 800mLof water in a 1000-mLvolumetric flask.Adjust with 1Mphosphoric acid to a pHof 3.0±0.05,dilute with water to volume,and mix.
Mobile phase—
Prepare a filtered and degassed mixture of Buffer solution,acetonitrile,and methanol (2:2:1).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard stock preparation—
Dissolve an accurately weighed quantity of USP Felodipine RSin methanol,and dilute quantitatively,and stepwise if necessary,with methanol to obtain a solution having a known concentration of about 2mg per mL.
Standard preparation—
Dilute an accurately measured volume of the Standard stock preparationquantitatively and stepwise with Mobile phaseto obtain a solution having a known concentration of about 0.02mg per mL.
Assay preparation—
Weigh and finely powder not fewer than 10Tablets.Transfer an accurately weighed portion of the powder,equivalent to about 10mg of felodipine,to a 100-mLvolumetric flask,add 40mLof acetonitrile and 20mLof methanol,and sonicate for 5minutes.Add about 30mLof Buffer solution,and shake by mechanical means for 30minutes.Allow the solution to cool to room temperature,dilute with Buffer solutionto volume,and mix.Centrifuge a portion of the solution at high speed for 15minutes.Transfer 10mLof the supernatant to a 50-mLvolumetric flask,dilute with Mobile phaseto volume,and mix.Pass a portion of this solution through a filter having a 0.5-µm or finer porosity,discarding the first 4mLof the filtrate.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 362-nm detector and a 4.6-mm ×15-cm column that contains packing L1.The flow rate is about 1mLper minute.Chromatograph theStandard preparation,and record the peak responses as directed for Procedure:the column efficiency is not less than 1500theoretical plates;and the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 40µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of felodipine (C18H19Cl2NO4)in the portion of Tablets taken by the formula:
500C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Felodipine RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(PA5)Pharmaceutical Analysis 5
USP28–NF23Page 808
Pharmacopeial Forum:Volume No.30(2)Page 482
Phone Number:1-301-816-8305