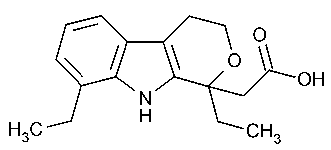
Etodolac
Pyrano[3,4-b]indole-1-acetic acid,1,8-diethyl-1,3,4,9-tetrahydro-(±)-.
(±)-1,8-Diethyl-1,3,4,9-tetrahydropyrano [3,4-b]indole-1-acetic acid [41340-25-4].
»Etodolac contains not less than 98.0percent and not more than 102.0percent of C17H21NO3,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Identification,Infrared Absorption á197Kñ.
Water,Method Iá921ñ:
not more than 0.5%.
Residue on ignition á281ñ:
not more than 0.1%.
Limit of chloride—
Dissolve 1.0g of Etodolac in 60mLof methanol,dilute with 10mLof water,add 20mLof 2Nnitric acid,and titrate with 0.01Nsilver nitrate determining the endpoint potentiometrically (see Titrimetry á541ñ):the limit is not more than 0.3mg per g.
Heavy metals,Method IIá231ñ:
0.001%.
Limit of alcohol and methanol—
Internal standard stock solution—
Dissolve suitable quantities of isopropyl alcohol in dimethylformamide to obtain a solution containing about 2.5µLper mL.
Internal standard solution—
Pipet 5.0mLof Internal standard stock solutioninto a 100-mLvolumetric flask.Dilute with dimethylformamide to volume,and mix.This solution contains 0.125µLof isopropyl alcohol.
Standard stock solution—
Transfer 5.0mLof methanol and 5.0mLof alcohol to a 200-mLvolumetric flask.Dilute with dimethylformamide to volume,and mix.Pipet 5.0mLof this solution into a 100-mLvolumetric flask,dilute with dimethylformamide to volume,and mix.
Standard solution—
Pipet 10.0mLof Standard stock solutionand 5.0mLof Internal standard stock solutioninto a 100-mLvolumetric flask.Dilute with dimethylformamide to volume,and mix.This solution contains 0.101mg of methanol and 0.099mg of alcohol per mL.
Test solution—
Transfer about 0.5g of Etodolac,accurately weighed,to a 10-mLflask.Pipet 5.0mLof Internal standard solutioninto the flask.Insert a stopper into the flask,and sonicate to dissolve.
Chromatographic system
(see Chromatography á621ñ)—The gas chromatograph is equipped with a flame-ionization detector and a 0.32-mm ×25-m fused silica capillary column coated with a 5-µm film of phase G36.The carrier gas is helium,with a split flow rate of 50mLper minute.The injection port temperature is maintained at 200 ,and the detector is maintained at 300
,and the detector is maintained at 300 .The column temperature is maintained at 45
.The column temperature is maintained at 45 for 5minutes,then programmed to increase at the rate of 30
for 5minutes,then programmed to increase at the rate of 30 per minute to 280
per minute to 280 and to maintain this temperature for 27minutes.Chromatograph the Standard solutionand the Test solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.6for methanol,0.8for alcohol,and 1.0for isopropyl alcohol;and the resolution,R,between peaks is not less than 1.
and to maintain this temperature for 27minutes.Chromatograph the Standard solutionand the Test solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.6for methanol,0.8for alcohol,and 1.0for isopropyl alcohol;and the resolution,R,between peaks is not less than 1.
Procedure—
Separately inject equal volumes (about 1µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the areas of the peak responses.Identify by their retention times any peaks present in the chromatogram obtained from the Test solutionthat correspond to those in the chromatogram obtained from the Standard solution.Calculate the percentages of C2H5OHand CH3OHin the portion of Etodolac taken by the formula:
500(C/W)(RU/RS),
in which Cis the concentration,in mg per mL,of C2H5OHor CH3OHin the Standard solution;Wis the weight,in mg,of Etodolac taken to prepare the Test solution;and RUand RSare the peak area ratios of the relevant analyte to the internal standard obtained from the Test solutionand the Standard solution,respectively:not more than 0.1%of each is found.
Chromatographic purity—
Solution A—
Mix 0.6mLof phosphoric acid with 100mLof water.
Solution B—
Mix 0.6mLof phosphoric acid with 100mLof acetonitrile.
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
System suitability solution—
Dissolve suitable quantities of USP Etodolac Related Compound A RSand USP Etodolac RSin acetonitrile to obtain a solution containing about 0.01mg per mLand 0.2mg per mL,respectively.
Test solution—
Transfer about 25mg of Etodolac,accurately weighed,to a 250-mLvolumetric flask,dissolve in and dilute with acetonitrile to volume,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 214-nm detector and a 4-mm ×25-cm column that contains packing L7.The flow rate is about 1mLper minute.The chromatograph is programmed as follows.
Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.8for etodolac related compound Aand 1.0for etodolac;the resolution,R,between etodolac related compound Aand etodolac is not less than 3;the tailing factor is not more than 2;and the relative standard deviation for replicate injections is not more than 3%.
| Time (minutes) |
Solution A
(%) |
Solution B
(%) |
Elution |
| 0-5 | 60 | 40 | isocratic |
| 5-35 | 60®20 | 40®80 | linear gradient |
| 60 | 40 | re-equilibration |
Procedure—
Inject a volume (about 20µL)of the Test solutioninto the chromatograph,record the chromatogram,and measure the peak responses.Calculate the percentage of each impurity in the portion of Etodolac taken by the formula:
100(ri/rs),
in which riis the peak response for each impurity;and rsis the sum of the responses of all of the peaks:not more than 0.5%of any individual impurity is found;and the total of all impurities is not more than 2.0%.
Assay—
Dissolve about 230mg of Etodolac in 60mLof methanol.Titrate with 0.1Ntetrabutylammonium hydroxide VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.1Ntetrabutylammonium hydroxide is equivalent to 28.74mg of C17H21NO3.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA2)Pharmaceutical Analysis 2
USP28–NF23Page 799
Phone Number:1-301-816-8139