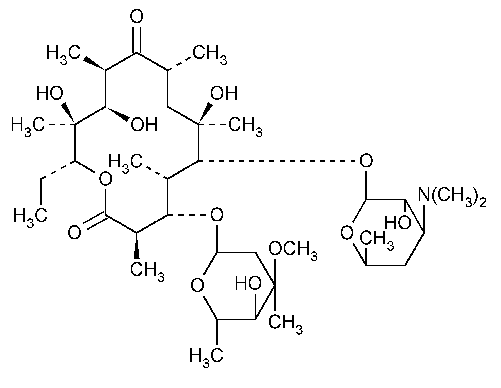
Erythromycin
Erythromycin.
(3R*,4S*,5S*,6R*,7R*,9R*,11R*,12R*,13S*,14R*)-4-[(2,6-Dideoxy-3-C-methyl-3-O-methyl-a-L-ribo-hexopyranosyl)oxy]-14-ethyl-7,12,13-trihydroxy-3,5,7,9,11,13-hexamethyl-6-[[3,4,6-trideoxy-3-(dimethylamino)-b-D-xylo-hexopyranosyl]oxy]oxacyclotetradecane-2,10-dione [114-07-8].
»Erythromycin consists primarily of erythromycin A(C37H67NO13).The sum of the percentages of erythromycin A,erythromycin B,and erythromycin Cis not less than 85.0percent and not more than 100.5percent,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
USP Reference standards á11ñ—
USP Erythromycin RS.USP Erythromycin B RS.USP Erythromycin C RS.USP Erythromycin Related Compound N RS.
Identification—
The IRabsorption spectrum of a solution of it containing 50mg per mL,previously dried at a pressure not exceeding 5mm of mercury at 60 for 3hours,in chloroform,determined in a 0.1-mm cell,exhibits maxima only at the same wavelengths as that of a similar preparation of USP Erythromycin RS,except in the region between 1980cm-1and 2050cm-1.
for 3hours,in chloroform,determined in a 0.1-mm cell,exhibits maxima only at the same wavelengths as that of a similar preparation of USP Erythromycin RS,except in the region between 1980cm-1and 2050cm-1.
Specific rotation á781Sñ:
between -71 and -78
and -78 ,determined after standing for 30minutes.
,determined after standing for 30minutes.
Test solution:
20mg per mL,in dehydrated alcohol.
Crystallinity á695ñ:
meets the requirements.
Water,Method Iá921ñ:
not more than 10.0%,20mLof methanol containing 10%of imidazole being used in place of methanol in the titration vessel.
Residue on ignition á281ñ:
not more than 0.2%.
Limit of thiocyanate—
Standard solutions—
Transfer about 100mg of potassium thiocyanate,previously dried at 105 for 1hour,cooled,and accurately weighed,to each of two 50-mLvolumetric flasks.Add about 20mLof methanol to each flask,swirl to dissolve,dilute with methanol to volume,and mix.Transfer 5.0mLof each of these stock solutions to separate 50-mLvolumetric flasks,dilute with methanol to volume,and mix.Transfer 5.0mLof each of these intermediate solutions to separate 50-mLlow-actinic volumetric flasks.To each flask add 1.0mLof ferric chloride TS,dilute with methanol to volume,and mix.[NOTE—Use these Standard solutions within 30minutes.]
for 1hour,cooled,and accurately weighed,to each of two 50-mLvolumetric flasks.Add about 20mLof methanol to each flask,swirl to dissolve,dilute with methanol to volume,and mix.Transfer 5.0mLof each of these stock solutions to separate 50-mLvolumetric flasks,dilute with methanol to volume,and mix.Transfer 5.0mLof each of these intermediate solutions to separate 50-mLlow-actinic volumetric flasks.To each flask add 1.0mLof ferric chloride TS,dilute with methanol to volume,and mix.[NOTE—Use these Standard solutions within 30minutes.]
Test solution—
Transfer about 100mg of Erythromycin,accurately weighed,to a 50-mLlow-actinic volumetric flask.Add 20mLof methanol,and swirl to dissolve.Add 1.0mLof ferric chloride TS,dilute with methanol to volume,and mix.[NOTE—Use this Test solution within 30minutes.]
Blank solution—
Transfer 1.0mLof ferric chloride TSto a 50-mLlow-actinic volumetric flask,dilute with methanol to volume,and mix.[NOTE—Use this Blank solution within 30minutes.]
Procedure—
Determine the absorbances of each Standard solutionand the Test solutionat the wavelength of maximum absorbance at about 492nm with a spectrophotometer,using the Blank solutionto zero the instrument.Calculate the suitability value,S,by the formula:
(A1/W1)(W2/A2),
in which A1and A2are the absorbance values obtained from the respective Standard solutions;and W1and W2are the weights,in mg,of the potassium thiocyanate taken to prepare the corresponding Standard solutions.In a suitable determination,the value,S,is not less than 0.985and not more than 1.015.Calculate the percentage of thiocyanate in the Erythromycin taken by the formula:
(58.08/97.18)(AU/WU)(0.5)[(W1/A1)+(W2/A2)],
in which 58.08and 97.18are the molecular weights of the thiocyanate moiety and of potassium thiocyanate,respectively;AUis the absorbance of the Test solution;WUis the weight,in mg,of Erythromycin taken to prepare the Test solution;and the other terms are as defined above:not more than 0.3%is found.
Limit of related substances—
Using the chromatograms of the Assay preparationand the Diluted standard preparationobtained in the Assay,calculate the percentage of any individual related substance observed having the greatest response,other than erythromycin A,erythromycin B,erythromycin C,and erythromycin Aenol ether,in the Erythromycin taken by the formula:
25(CP/W)(ri/rS),
in which Cis the concentration,in mg per mL,of USP Erythromycin RSin the Diluted standard preparation;Pis the designated percentage of erythromycin Ain the USP Erythromycin RS;Wis the weight,in mg,of Erythromycin taken to prepare the Assay preparation;riis the peak response of an individual related substance,other than erythromycin A,erythromycin B,erythromycin C,or erythromycin Aenol ether,observed in the chromatogram obtained from the Assay preparation;and rSis the erythromycin Apeak response in the chromatogram obtained from the Diluted standard preparation:not more than 3.0%of any individual related substance is found.Calculate the percentage of erythromycin Aenol ether in the Erythromycin taken by the formula:
(25/11)(CP/W)(rE/rS),
in which 11is the response factor for erythromycin Aenol ether in relation to that of erythromycin A;rEis the peak response of the erythromycin Aenol ether peak observed in the chromatogram obtained from the Assay preparation;and the other terms are as defined above:not more than 3.0%of erythromycin Aenol ether is found.The percentage of erythromycin Bobtained in the Assayis not more than 12.0%;and the percentage of erythromycin Cobtained in the Assayis not more than 5.0%.
Assay—
Solution A—
Dissolve 1.75g of dibasic potassium phosphate in 50mLof water,adjust with diluted phosphoric acid (1in 10)or 0.2Nsodium hydroxide to a pHof 9.0,add 400mLof water,165mLof tertiary butyl alcohol,and 30mLof acetonitrile.Dilute with water to 1000mL,and mix.
Mobile phase—
Prepare a mixture of Solution A,acetonitrile,and water (5:2:1).Make any necessary adjustments (see System Suitabilityunder Chromatography á621ñ).
Diluent—
Prepare a mixture of pH7.0buffer (see under Buffer Solutionsin the section Reagents,Indicators,and Solutions)and methanol (15:1).
pH3.5Buffer—
Adjust 20mLof pH7.0buffer (see under Buffer Solutionsin the section Reagents,Indicators,and Solutions)with phosphoric acid to a pHof 3.5.
NOTE—Use the following solutions promptly,or within 1day if stored in a refrigerator.
Standard preparation—
Transfer about 100mg of USP Erythromycin RS,accurately weighed,to a 25-mLvolumetric flask,add 5mLof methanol,swirl to dissolve,dilute with Diluentto volume,and mix.
Diluted standard preparation—
Transfer 3.0mLof the Standard preparationto a 100-mLvolumetric flask,dilute with Diluentto volume,and mix.This solution contains about 0.12mg of USP Erythromycin RSper mL.
Erythromycins Band Cstandard solution—
Transfer about 5mg each of USP Erythromycin B RSand USP Erythromycin C RS,both accurately weighed,to a 25-mLvolumetric flask,add 5mLof methanol,swirl to dissolve,dilute with Diluentto volume,and mix.
Resolution solution—
Transfer about 2mg of USP Erythromycin Related Compound N RSto a 10-mLvolumetric flask,add 0.4mLof Standard preparation,dilute with Erythromycins Band Cstandard solutionto volume,and mix.
Erythromycin Aenol ether retention time solution—
Dissolve about 10mg of USP Erythromycin RSin 2mLof methanol.Add 10mLof pH3.5Buffer,mix,and allow to stand for about 30minutes.
Assay preparation—
Transfer about 100mg of Erythromycin,accurately weighed,to a 25-mLvolumetric flask,add 5mLof methanol,and swirl to dissolve.Dilute with Diluentto volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 215-nm detector and a 4.6-mm ×25-cm column that contains packing L21(1000Å)and is maintained at a constant temperature of about 65 .The flow rate is about 2mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.56for erythromycin related compound N(N-demethylerythromycin A),0.61for erythromycin C,1.0for erythromycin A,and 1.6for erythromycin B;and the resolution,R,between erythromycin related compound Nand erythromycin Cis not less than 0.8,and between erythromycin related compound Nand erythromycin Anot less than 5.5.Chromatograph the Erythromycin Aenol ether retention time solution,and record the peak responses as directed for Procedure:the retention time of the erythromycin Aenol ether peak is about 3.2relative to that of the erythromycin Apeak as observed in the chromatogram obtained from the Resolution solution.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
.The flow rate is about 2mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.56for erythromycin related compound N(N-demethylerythromycin A),0.61for erythromycin C,1.0for erythromycin A,and 1.6for erythromycin B;and the resolution,R,between erythromycin related compound Nand erythromycin Cis not less than 0.8,and between erythromycin related compound Nand erythromycin Anot less than 5.5.Chromatograph the Erythromycin Aenol ether retention time solution,and record the peak responses as directed for Procedure:the retention time of the erythromycin Aenol ether peak is about 3.2relative to that of the erythromycin Apeak as observed in the chromatogram obtained from the Resolution solution.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 100µL)of the Standard preparation,the Diluted standard preparation,the Erythromycins Band Cstandard solution,and the Assay preparationinto the chromatograph,record the chromatograms for a period of time that is adequate to include the erythromycin Aenol ether peak,if present,as determined in the chromatogram obtained from the Erythromycin Aenol ether retention time solution(about five times the retention time of the main erythromycin Apeak).Measure the areas of the peak responses.Calculate the percentage of erythromycin Ain the portion of Erythromycin taken by the formula:
25(CAP/W)(rU/rA),
in which CAis the concentration,in mg per mL,of USP Erythromycin RSin the Standard preparation;Pis the designated percentage of erythromycin Ain USP Erythromycin RS;Wis the quantity,in mg,of Erythromycin taken to prepare the Assay preparation;and rUand rAare the erythromycin Apeak responses in the chromatograms obtained from the Assay preparationand the Standard preparation,respectively.Calculate the percentages of erythromycin Band erythromycin Cin the portion of Erythromycin taken by the formula:
25(CSP/W)(rU/rS),
in which CSis the concentration,in mg per mL,of the relevant USP Reference Standard in the Erythromycins Band Cstandard solution;Pis the designated percentage of erythromycin Bor erythromycin Cin the relevant USP Reference Standard;Wis the quantity,in mg,of Erythromycin taken to prepare the Assay preparation;and rUand rSare the peak responses of the relevant analyte in the chromatograms obtained from the Assay preparationand the Erythromycins Band Cstandard solution,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 757
Phone Number:1-301-816-8335