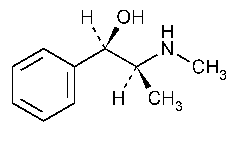
Ephedrine
Benzenemethanol,a-[1-(methylamino)ethyl]-,[R-(R*,S*)]-.
(-)-Ephedrine [299-42-3].
Hemihydrate 174.24 [50906-05-3].
»Ephedrine is anhydrous or contains not more than one-half molecule of water of hydration.It contains not less than 98.5percent and not more than 100.5percent of C10H15NO,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight,light-resistant containers,in a cold place.
Labeling—
Label it to indicate whether it is hydrous or anhydrous.Where the quantity of Ephedrine is indicated in the labeling of any preparation containing Ephedrine,this shall be understood to be in terms of anhydrous Ephedrine.
Identification—
Weigh accurately about 100mg,and add by buret the exact volume of 0.1Nsulfuric acid,determined in the Assay,to neutralize it.Dilute with water in a volumetric flask to 25mL.Mix 2mLwith 10mLof alcohol,and evaporate on a steam bath with the aid of a current of air to dryness:the residue so obtained responds to Identificationtest Aunder Ephedrine Sulfate.
Specific rotation á781Sñ:
between -40.3 and -43.3
and -43.3 .
.
Test solution:
25mg per mL,in 0.6Nhydrochloric acid.
Water,Method Ib á921ñ:
between 4.5%and 5.5%,for hydrated Ephedrine;not more than 0.5%for anhydrous Ephedrine.
Residue on ignition á281ñ:
not more than 0.1%.
Chloride á221ñ—
Asolution of 500mg shows no more chloride than corresponds to 0.20mLof 0.020Nhydrochloric acid (0.030%).
Sulfate—
Dissolve 100mg in 40mLof water,and add 1mLof 3Nhydrochloric acid and 1mLof barium chloride TS:no turbidity develops within 10minutes.
Ordinary impurities á466ñ—
Test solution:
methanol.
Standard solution:
methanol.
Eluant:
a mixture of isopropyl alcohol,3.6Mammonium hydroxide,and chloroform (80:15:5).
Visualization:
1,followed by 4.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 500mg of Ephedrine,accurately weighed,in 10mLof neutralized alcohol,and add 5drops of methyl red TSand 40.0mLof 0.1Nhydrochloric acid VS.Titrate the excess acid with 0.1Nsodium hydroxide VS.Perform a blank determination (see Residual Titrations á541ñ).Each mLof 0.1Nhydrochloric acid is equivalent to 16.52mg of C10H15NO.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 736
Pharmacopeial Forum:Volume No.30(3)Page 839
Phone Number:1-301-816-8379