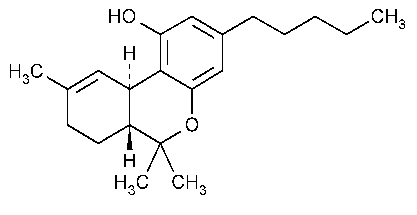
Dronabinol
6H-Dibenzo[b,d]pyran-1-ol,6a,7,8,10a-tetrahydro-6,6,9-trimethyl-3-pentyl-,(6aR-trans)-.
(6aR,10aR)-6a,7,8,10a-Tetrahydro-6,6,9-trimethyl-3-pentyl-6H-dibenzo[b,d]-pyran-1-ol [1972-08-3].
»Dronabinol is D9-tetrahydrocannabinol.It contains not less than 95.0percent of C21H30O2.
Packaging and storage—
Preserve in tight,light-resistant glass containers in inert atmosphere.Store in a cool place.
Identification—
A:
The retention time of the major peak (other than that due to the internal standard)in the chromatogram of the Assay preparationcorresponds to that of the Standard preparation,as obtained in the Assay.
B:
[NOTE—Since the visualizing agent used in this test is specific for phenols,solution preparations containing diphenyl phthalate and/or sesame oil may be used without interference.]
Visualizing agent—
Transfer about 100mg of Fast Blue Bsalt to a suitable flask containing about 100mLof methanol,stir for about 5minutes,and allow to settle.Decant the clear liquid into the sprayer reservoir.[NOTE—Prepare fresh daily.]
Procedure—
Apply separately 10µLeach of the Identification preparationand the Test preparationto a suitable thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel.Allow the spots to dry,and develop the plate in a chromatographic chamber that has been equilibrated (for about 2minutes)with vapors from a solvent mixture of n-hexane and methylene chloride (1:1)until the solvent front has moved about 10cm.Remove the plate from the developing chamber,quickly mark the solvent front,and allow the plate to dry at room temperature for about 5minutes.Spray the plate with the Visualizing agentuntil it is uniformly damp (not saturated).Heat the plate at about 80 until the spots are developed:the color and RFvalue of the spot from the Test preparationcorrespond to those obtained from the Identification preparation.
until the spots are developed:the color and RFvalue of the spot from the Test preparationcorrespond to those obtained from the Identification preparation.
Limit of D8-tetrahydrocannabinol—
Mobile phase
,System suitability solution,Standard preparation,and Chromatographic system—Proceed as directed in the Assay.
D8-Tetrahydrocannabinol solution—
Dilute an accurately measured volume of USPD8-Tetrahydrocannabinol RSquantitatively,and stepwise if necessary,with dehydrated alcohol to obtain a solution having a known concentration of about 4µg per mL.
Test solution—
Use the Assay preparation.
Procedure—
Separately inject equal volumes (about 20µL)of the D8-Tetrahydrocannabinol solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for all of the peaks.Calculate the percentage of D8-tetrahydrocannabinol in the portion of Dronabinol taken by the formula:
10,000(C/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USPD8-Tetrahydrocannabinol RSin the D8-Tetrahydrocannabinol solution;Wis the weight,in mg,of dronabinol in the portion of Dronabinol taken to prepare the Test solution;and rUand rSare the D8-tetrahydrocannabinol peak responses obtained from the Test solutionand the D8-Tetrahydrocannabinol solution,respectively:not more than 2.0%is found.
Assay—
Mobile phase—
Prepare a filtered and degassed mixture of methanol,water,and tetrahydrofuran (71:24:5),making adjustments,if necessary (see System Suitabilityunder Chromatography á621ñ).
System suitability solution—
Mix accurately measured volumes of USPD9-Tetrahydrocannabinol RSand USPD8-Tetrahydrocannabinol RSin dehydrated alcohol to obtain a solution having a known concentration of about 0.5mg per mLof each component.
Standard preparation—
Dissolve an accurately measured volume of USPD9-Tetrahydrocannabinol RSin dehydrated alcohol to obtain a solution having a known concentration of about 0.2mg per mL.
Assay preparation—
Transfer about 20mg of Dronabinol,accurately weighed,to a 100-mLvolumetric flask,dissolve in dehydrated alcohol,dilute with dehydrated alcohol to volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 228-nm detector,a 4.6-×30-mm guard column that contains 5-µm packing L1,and a 4.6-mm ×15-cm analytical column that contains 3-µm packing L1.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the relative retention times are about 1.0for D9-tetrahydrocannabinol and 1.14for D8-tetrahydrocannabinol;the resolution,R,between dronabinol and D8-tetrahydrocannabinol is not less than 2.0;and the tailing factor of the D9-tetrahydrocannabinol peak is not more than 2.0.Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not greater than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for all of the peaks.Calculate the quantity,in mg,of C21H30O2in the portion of Dronabinol taken by the formula:
100C(rU/rS),
in which Cis the concentration,in mg per mL,of USPD9-Tetrahydrocannabinol RSin the Standard preparation;and rUand rSare the dronabinol peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 703
Phone Number:1-301-816-8251