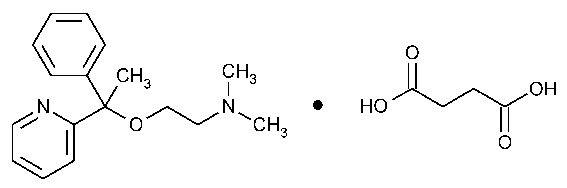
Doxylamine Succinate
Ethanamine,N,N-dimethyl-2-[1-phenyl-1-(2-pyridinyl)ethoxy]-,butanedioate (1:1).
2-[a-[2-(Dimethylamino)ethoxy]-a-methylbenzyl]pyridine succinate (1:1) [562-10-7].
»Doxylamine Succinate contains not less than 98.0percent and not more than 101.0percent of C17H22N2O·C4H6O4,calculated on the dried basis.
Packaging and storage—
Preserve in well-closed,light-resistant containers.
Identification—
A:
Ultraviolet absorption á197Uñ—
Solution:
20µg per mL.
Medium:
0.1Nhydrochloric acid.
Absorptivities at 262nm,calculated on the dried basis,do not differ by more 3.0%.
B:
It meets the requirements under Identification—Organic Nitrogenous Bases á181ñ.
C:
Dissolve about 500mg in 5mLof water,and add a slight excess of 6Nammonium hydroxide.Extract the liberated doxylamine with several portions of ether,discard the ether extracts,and evaporate the aqueous solution on a steam bath to dryness.Add 2mLof 3Nhydrochloric acid,and again evaporate on the steam bath to dryness.Cool,add about 10mLof ether,allow to stand for a few minutes,and decant the clear supernatant.Evaporate the ether solution to dryness,and dry the residue at 105 for 30minutes:the succinic acid so obtained melts between 184
for 30minutes:the succinic acid so obtained melts between 184 and 188
and 188 ,the procedure for Class Ibeing used (see Melting Range or Temperature á741ñ).
,the procedure for Class Ibeing used (see Melting Range or Temperature á741ñ).
Melting range,Class Iá741ñ:
between 103 and 108
and 108 ,but the range between beginning and end of melting does not exceed 3
,but the range between beginning and end of melting does not exceed 3 .
.
Loss on drying á731ñ—
Dry it in vacuum over phosphorus pentoxide for 5hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.1%.
Volatile related compounds—
Dissolve 650mg in 20mLof 0.1Nhydrochloric acid in a separator.Render the solution alkaline with 2.5Nsodium hydroxide,and immediately extract with four 25-mLportions of ether,filtering each extract through an ether-saturated pledget of cotton.Evaporate the combined ether extracts on a water bath with the aid of a current of air to dryness at a temperature not exceeding 50 ,and dissolve the residue in 5mLof alcohol.Inject about 1µLof this solution into a suitable gas chromatograph (see Chromatography á621ñ)equipped with a flame-ionization detector.Under typical conditions,the instrument contains a 2-m ×4-mm glass column containing 5%packing G16and 5%packing G12on 60-to 80-mesh S1A.The column is maintained at about 212
,and dissolve the residue in 5mLof alcohol.Inject about 1µLof this solution into a suitable gas chromatograph (see Chromatography á621ñ)equipped with a flame-ionization detector.Under typical conditions,the instrument contains a 2-m ×4-mm glass column containing 5%packing G16and 5%packing G12on 60-to 80-mesh S1A.The column is maintained at about 212 ,the injection port and detector block are maintained at about 250
,the injection port and detector block are maintained at about 250 ,and dry helium is used as the carrier gas.The total relative area of all extraneous peaks (except that of the solvent peak)does not exceed 2.0%,and the relative area of any individual extraneous peak does not exceed 1.0%.
,and dry helium is used as the carrier gas.The total relative area of all extraneous peaks (except that of the solvent peak)does not exceed 2.0%,and the relative area of any individual extraneous peak does not exceed 1.0%.
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Dissolve about 500mg of Doxylamine Succinate,accurately weighed,in 80mLof glacial acetic acid.Add crystal violet TS,and titrate with 0.1Nperchloric acid VSto an emerald-green endpoint.Perform a blank determination,and make any necessary correction.Each mLof 0.1Nperchloric acid is equivalent to 19.42mg of C17H22N2O·C4H6O4.
Auxiliary Information—
Staff Liaison:Karen A Russo,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 702
Phone Number:1-301-816-8379