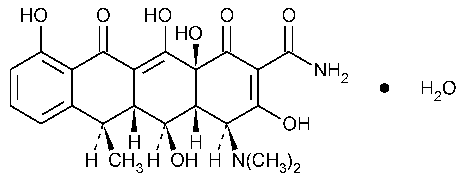
Doxycycline
2-Naphthacenecarboxamide,4-(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-,[4S-(4a,4aa,5a,5aa,6a,12aa)]-,monohydrate.
4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-de monohydrate [17086-28-1].
Anhydrous 444.44 [564-25-0].
»Doxycycline has a potency equivalent to not less than 880µg and not more than 980µg of C22H24N2O8per mg.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
Dissolve a suitable quantity in methanol to obtain a Test Solutioncontaining 1mg of doxycycline per mL,and proceed as directed for Method IIunder Identification—Tetracyclines á193ñ.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 5.0and 6.5,in an aqueous suspension containing 10mg per mL.
Water,Method Iá921ñ:
between 3.6%and 4.6%.
Related compounds—
System suitability solution—
Prepare as directed for the Resolution solutionin the Assayunder Doxycycline Hyclate.
Methacycline standard stock solution,Standard solution 1,Standard solution 2,andChromatographic system—
Prepare as directed for the Related compoundstest under Doxycycline Hyclate.
Procedure—
Proceed as directed for the Related compoundstest under Doxycycline Hyclate.Calculate the percentage of methacycline in the portion of Doxycycline taken by the formula:
5000(CM/W)(rU/rM),
in which CMis the concentration,in mg per mL,of USP Methacycline Hydrochloride RSin Standard solution 2;Wis the weight,in mg,of Doxycycline taken to prepare the Test solution;and rUand rMare the methacycline peak responses obtained from the Test solutionand Standard solution 2,respectively.Not more than 2%of methacycline is found.Calculate the percentage of each related compound,other than methacycline,in the portion of Doxycycline taken by the formula:
5000(CS/W)(ri/rS),
in which CSis the concentration,in mg per mL,of USP Doxycycline Hyclate RSin Standard solution 2;Wis the weight,in mg,of Doxycycline taken to prepare the Test solution;riis the peak response for each impurity obtained from the Test solution;and rSis the doxycycline peak response obtained from Standard solution 2.Not more than 0.5%of any impurity eluting before methacycline is found;not more than 2%of 6-epidoxycycline is found;and not more than 0.5%of any impurity eluting after the main doxycycline peak is found.
Assay—
Mobile phase,Diluent,Resolution solution,Standard preparation,andChromatographic system—
Prepare as directed in the Assayunder Doxycycline Hyclate.
NOTE—Throughout the following sections,protect the Standard preparationand the Assay preparationfrom light.
Assay preparation—
Transfer about 55mg of Doxycycline,accurately weighed,to a 50-mLvolumetric flask,add 12mLof 0.1Nhydrochloric acid,swirl to dissolve,dilute with Diluentto volume,and mix.Filter through a filter of 0.5µm or finer porosity.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in µg,of doxycycline (C22H24N2O8)in each mg of the Doxycycline taken by the formula:
50(CP/W)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Doxycycline Hyclate RSin the Standard preparation,Pis the potency,in µg of doxycycline per mg,of USP Doxycycline Hyclate RS,Wis the weight,in mg,of Doxycycline taken to prepare the Assay preparation,and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 697
Pharmacopeial Forum:Volume No.26(6)Page 1544
Phone Number:1-301-816-8335