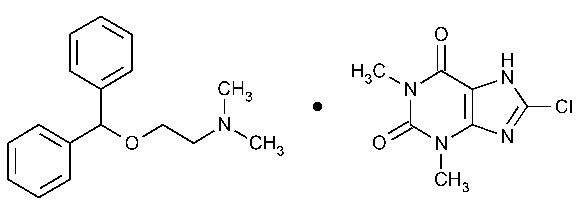
Dimenhydrinate
1H-Purine-2,6-dione,8-chloro-3,7-dihydro-1,3-dimethyl-,compd.with 2-(diphenylmethoxy)-N,N-dimethylethanamine (1:1).
8-Chlorotheophylline,compound with 2-(diphenylmethoxy)-N,N-dimethylethylamine (1:1) [523-87-5].
»Dimenhydrinate contains not less than 53.0percent and not more than 55.5percent of diphenhydramine (C17H21NO),and not less than 44.0percent and not more than 47.0percent of 8-chlorotheophylline (C7H7ClN4O2),calculated on the dried basis.
Packaging and storage—
Preserve in well-closed containers.
Identification—
A:
It meets the requirements under Identification—Organic Nitrogenous Bases á181ñ.
B:
Dissolve about 250mg in 15mLof diluted alcohol,add 15mLof water and 2mLof 2Nsulfuric acid,and cool for 30minutes.Scratch the inside of the container to facilitate crystallization.Filter the mixture,wash the crystals with a few mLof ice-cold water,and dry the crystals:the 8-chlorotheophylline melts between 300 and 305
and 305 with decomposition.
with decomposition.
C:
To about 10mg of the 8-chlorotheophylline obtained in Identificationtest B,contained in a porcelain dish,add 1mLof hydrochloric acid and 100mg of potassium chlorate,evaporate on a steam bath to dryness,and invert the dish over a vessel containing a few drops of ammonia TS:the residue acquires a purple color,which is destroyed by solutions of fixed alkalies.
D:
Mix about 50mg of the 8-chlorotheophylline obtained in Identificationtest Bwith about 500mg of sodium peroxide in a nickel crucible,and heat until the mass is well sintered.Dissolve the melt in 20mLof water,acidify with 2Nnitric acid,filter if necessary,and add 1mLof silver nitrate TS:a curdy,white precipitate is formed,and it is soluble in 6Nammonium hydroxide and reappears upon acidification with nitric acid.
Loss on drying á731ñ—
Dry it in vacuum over phosphorus pentoxide for 24hours:it loses not more than 0.5%of its weight.
Residue on ignition á281ñ:
not more than 0.3%.
Bromide and iodide—
Mix in a test tube 100mg of Dimenhydrinate,50mg of sodium nitrite,and 10mLof chloroform.Add 10mLof 3Nhydrochloric acid,insert the stopper in the tube,and shake:the chloroform remains colorless.
Chloride—
When the ammoniacal filtrate from the precipitation of silver chlorotheophylline in the Assay for 8-chlorotheophyllineis acidified preparatory to titration,the solution shows not more than a faint opalescence.
Organic volatile impurities,Method Vá467ñ:
meets the requirements.
Solvent—
Use dimethyl sulfoxide.
Assay for diphenhydramine—
Dissolve about 150mg of Dimenhydrinate,accurately weighed,in 75mLof glacial acetic acid,and titrate with 0.05Nperchloric acid VS,determining the endpoint potentiometrically.Perform a blank determination,and make any necessary correction.Each mLof 0.05Nperchloric acid is equivalent to 12.77mg of diphenhydramine (C17H21NO).
Assay for 8-chlorotheophylline—
Place about 800mg of Dimenhydrinate,accurately weighed,in a 200-mLvolumetric flask,add 50mLof water,3mLof 6Nammonium hydroxide,and 6mLof ammonium nitrate solution (1in 10),and warm the mixture on a steam bath for 5minutes.Add 25.0mLof 0.1Nsilver nitrate VS,mix,and warm on a steam bath for 15minutes with frequent shaking.Cool,dilute with water to volume,mix,and allow the precipitate to settle.Filter through a dry filter paper,discarding the first 20mLof the filtrate.Pipet 100mLof the filtrate into a 250-mLflask,acidify with nitric acid,and add an excess of 3mLof the acid.Add 2mLof ferric ammonium sulfate TS,and titrate the excess silver nitrate with 0.1Nammonium thiocyanate VS.Each mLof 0.1Nsilver nitrate is equivalent to 21.46mg of C7H7ClN4O2.
Auxiliary Information—
Staff Liaison:Elena Gonikberg,Ph.D.,Scientist
Expert Committee:(PA4)Pharmaceutical Analysis 4
USP28–NF23Page 658
Pharmacopeial Forum:Volume No.29(5)Page 1466
Phone Number:1-301-816-8251