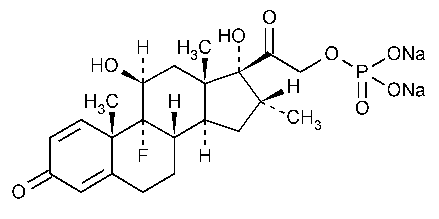
Dexamethasone Sodium Phosphate
Pregna-1,4-diene-3,20-dione,9-fluoro-11,17-dihydroxy-16-methyl-21-(phosphonooxy)-,disodium salt,(11b,16a)-.
9-Fluoro-11b,17,21-trihydroxy-16a-methylpregna-1,4-diene-3,20-dione 21-(dihydrogen phosphate)disodium salt [2392-39-4].
»Dexamethasone Sodium Phosphate contains not less than 97.0percent and not more than 102.0percent of C22H28FNa2O8P,calculated on the water-free and alcohol-free basis.
Packaging and storage—
Preserve in tight containers.
Identification—
A:
pH9Buffer with magnesium—Mix 3.1g of boric acid and 500mLof water in a 1-Lvolumetric flask,add 21mLof 1Nsodium hydroxide and 10mLof 0.1Mmagnesium chloride,dilute with water to volume,and mix.
Alkaline phosphatase solution—
Transfer 95±5mg of alkaline phosphatase enzyme to a 50-mLvolumetric flask,dissolve by adding pH9Buffer with magnesiumto volume,and mix.Prepare this solution fresh daily.
Standard solution—
Weigh 15mg of USP Dexamethasone RSinto a 5-mLvolumetric flask.Dissolve in and dilute with ethyl acetate to volume.[NOTE—Sonication may be required to ensure dissolution.]
Test solution—
Weigh 20mg of Dexamethasone Sodium Phosphate into a 15-mLcentrifuge tube.Add 5.0mLof Alkaline phosphatase solution,shake vigorously,and allow to stand for 30minutes.Add 5.0mLof ethyl acetate,shake vigorously,centrifuge,and use the upper,ethyl acetate layer.
Procedure—
Apply 10-µLportions of the Test solutionand the Standard solutionto a thin-layer chromatographic plate (see Chromatography á621ñ)coated with a 0.25-mm layer of chromatographic silica gel mixture.Develop the chromatogram in a mobile phase consisting of a mixture of chloroform,methanol,and water (180:15:1)to a distance of three-fourths of the length of the plate.Air-dry the plate and observe under short-wavelength UVlight:the RFvalue of the principal spot obtained from the Test solutioncorresponds to that obtained from the Standard solution.
B:
The residue from the ignition of it meets the requirements of the tests for Phosphate á191ñand for Sodium á191ñ.
Specific rotation á781Sñ:
between +74 and +82
and +82 ,calculated on the water-free and alcohol-free basis.
,calculated on the water-free and alcohol-free basis.
Test solution:
10mg per mL,in water.
pHá791ñ:
between 7.5and 10.5,in a solution (1in 100).
Water,Method Iá921ñ—
Determine the water content.The sum of the percentages of water content,and alcohol content,determined as directed in the test for Alcohol,does not exceed 16.0%.
Limit of phosphate ions—
Standard phosphate solution—
Dissolve 143.3mg of dried monobasic potassium phosphate,KH2PO4,in water to make 1000.0mL.This solution contains the equivalent of 0.10mg of phosphate (PO4)in each mL.
Phosphate reagent A—
Dissolve 5g of ammonium molybdate in 1Nsulfuric acid to make 100mL.
Phosphate reagent B—
Dissolve 350mg of p-methylaminophenol sulfate in 50mLof water,add 20g of sodium bisulfite,mix to dissolve,and dilute with water to 100mL.
Procedure—
Dissolve about 50mg of Dexamethasone Sodium Phosphate,accurately weighed,in a mixture of 10mLof water and 5mLof 2Nsulfuric acid contained in a 25-mLvolumetric flask,by warming if necessary.Add 1mLeach of Phosphate reagent Aand Phosphate reagent B,dilute with water to 25mL,mix,and allow to stand at room temperature for 30minutes.Similarly and concomitantly,prepare a standard solution,using 5.0mLof Standard phosphate solutioninstead of the 50mg of the substance under test.Concomitantly determine the absorbances of both solutions in 1-cm cells at 730nm,with a suitable spectrophotometer,using water as the blank.The absorbance of the test solution is not more than that of the standard solution.The limit is 1.0%of phosphate (PO4).
Limit of free dexamethasone—
Mobile phase—
Prepare a solution containing 7.5mLof triethylamine in 1liter of water.Adjust by the addition of phosphoric acid to a pHof 5.4.Prepare a filtered and degassed mixture of 74parts of the resulting solution with 26parts of methanol.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of USP Dexamethasone Phosphate RSin Mobile phaseto obtain a solution containing about 0.5mg per mL.Prepare a second solution by dissolving an accurately weighed quantity of USP Dexamethasone RSin a mixture of methanol and water (1:1)to obtain a solution containing about 50µg per mL.Transfer 10.0mLof the first solution and 1.0mLof the second solution to a 100-mLvolumetric flask.Dilute with Mobile phaseto volume,and mix to obtain a solution having known concentrations of 50µg of USP Dexamethasone Phosphate RSper mLand 0.5µg of USP Dexamethasone RSper mL.
Test solution—
Transfer about 50mg of Dexamethasone Sodium Phosphate,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.Further dilute 5.0mLof this solution with Mobile phaseto 50.0mL.
System suitability solution—
Prepare a solution in Mobile phasecontaining in each mL0.05mg of USP Dexamethasone Phosphate RSand 0.02mg of USP Dexamethasone RS.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.5-mm ×25-cm column that contains 5-µm packing L11.The flow rate is about 1.2mLper minute.Chromatograph the Standard solutionand the System suitability solution,record the peak responses as directed for Procedure,and determine the chromatographic characteristics from chromatograms obtained from the System Suitability:the column efficiency determined from the analyte peak is not less than 900theoretical plates;the tailing factor for the analyte peak is not more than 1.6;the resolution,R,between dexamethasone phosphate and dexamethasone is not less than 1.8;and the relative standard deviation for replicate injections is not more than 1.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the responses for the dexamethasone peaks.Calculate the quantity,in µg,of dexamethasone (C22H29FO5)in the portion of Dexamethasone Sodium Phosphate taken by the formula:
1000C(rU/rS),
in which Cis the concentration,in µg per mL,of USP Dexamethasone RSin the Standard solution;and rUand rSare the peak responses obtained from the Test solutionand the Standard solution,respectively:not more than 1.0%is found.
Chromatographic purity—
Acetate buffer—
Dissolve 7g of ammonium acetate in 1liter of water,adjust with glacial acetic acid to a pHof 4.0,and mix.
Solution A—
Prepare a filtered and degassed mixture of methanol,water,and Acetate buffer(7:7:6).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Solution B—
Prepare a filtered and degassed mixture of methanol and Acetate buffer(7:3).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.
Test solution—
Transfer about 25mg of Dexamethasone Sodium Phosphate,accurately weighed,to a 25-mLvolumetic flask,dissolve in and dilute with Solution Ato volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column that contains packing L7.The flow rate is about 1mLper minute.The column temperature is maintained at 40 .The chromatograph is programmed as follows.
.The chromatograph is programmed as follows.
Chromatograph the Test solution,and record the peak responses as directed for Procedure:the resolution between the major peak and the nearest impurity is not less than 1.0;and the relative standard deviation for replicate injections is not more than 4.0%.
| Time (minutes) | Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 90 | 10 | equilibration |
| 0–3.5 | 90 | 10 | isocratic |
| 3.5–23.5 | 90®60 | 10®40 | linear gradient |
| 23.5–34.5 | 60®5 | 40®95 | linear gradient |
| 34.5–59.5 | 5 | 95 | isocratic |
| 59.5–60 | 5®90 | 95®10 | linear gradient |
Procedure—
Separately inject equal volumes (about 15µL)of the Test solutioninto the chromatograph,record the chromatogram,and measure the peak responses.Calculate the percentage of each impurity in the portion of Dexamethasone Sodium Phosphate taken by the formula:
100(ri/rs),
in which riis the peak response for each impurity;and rsis the sum of the responses of all peaks:not more than 1.0%of any individual impurity is found,and not more than 2.0%of total impurities is found.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Alcohol content,Method IIá611ñ—
Proceed as directed in the chapter except to use column packing S8and to use the following modifications.
Internal standard solution—
Pipet 1mLof isopropyl alcohol into a 100-mLvolumetric flask,add water to volume,and mix.
Standard stock solution—
Prepare a solution of alcohol in water (1in 50).Determine the specific gravity at 25 (see Specific á841ñ),and obtain the percentage of C2H5OHby reference to the Alcoholometric Tablein the section Reference Tables.
(see Specific á841ñ),and obtain the percentage of C2H5OHby reference to the Alcoholometric Tablein the section Reference Tables.
Standard solution—
Into a 10-mLvolumetric flask pipet 4mLof Standard stock solutionand 5mLof Internal standard solution,add water to volume,and mix.Inject 2µLof this solution into the gas chromatograph.
Test solution—
Transfer about 500mg of Dexamethasone Sodium Phosphate,accurately weighed,into a 10-mLvolumetric flask.Pipet 5mLof Internal standard solutioninto the flask,and mix to dissolve.Add water to volume,and mix.Inject 2µLof this solution into the gas chromatograph.
Calculation—
Calculate the percentage of alcohol in the Dexamethasone Sodium Phosphate taken by the formula:
4(S/W)(Z/Y),
in which Sis the percentage of alcohol in the Standard stock solution;Wis the weight,in g,of Dexamethasone Sodium Phosphate used in the Test solution;and Yand Zare the ratios of the alcohol peak heights to the internal standard peak heights for the Standard solutionand the Test solution,respectively.The content of C2H5OHis not more than 8.0%.
Assay—
Buffer solution—
Dissolve 7.0g of ammonium acetate in 1liter of water,adjust with glacial acetic acid to a pHof 4.00±0.05,and mix.
Solution A—
Prepare a filtered and degassed mixture of methanol,water,and Buffer solution(350:350:300).
Solution B—
Prepare a filtered and degassed mixture of methanol and Buffer solution(700:300).
Mobile phase—
Use variable mixtures of Solution Aand Solution Bas directed for Chromatographic system.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard preparation—
Dissolve an accurately weighed quantity of USP Dexamethasone Phosphate RSin Solution Ato obtain a solution having a known concentration of about 0.92mg per mL.
Assay preparation—
Dissolve an accurately weighed quantity of Dexamethasone Sodium Phosphate in Solution A,and mix to obtain a solution having a concentration of about 1.0mg per mL.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column that contains packing L7.The column temperature is maintained at about 40 .The flow rate is about 1.0mLper minute.The chromatograph is programmed as follows.
.The flow rate is about 1.0mLper minute.The chromatograph is programmed as follows.
Chromatograph the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation is not more than 2.0%.Chromatograph the Assay preparation,and record the peak responses as directed for Procedure:the resolution,R,between dexamethasone phosphate and the nearest impurity eluting after it is not less than 1.0.
| Time (minutes) | Solution A
(%) |
Solution B
(%) |
Elution |
| 0 | 90 | 10 | equilibration |
| 0–3.5 | 90 | 10 | isocratic |
| 3.5–24 | 90®60 | 10®40 | linear gradient |
| 24–35 | 60®5 | 40®95 | linear gradient |
| 35–60 | 5 | 95 | isocratic |
| 60–60.1 | 5®90 | 95®10 | linear gradient |
| 60.1–65 | 90 | 10 | isocratic |
Procedure—
Separately inject equal volumes (about 15µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the areas for the major peaks.Calculate the quantity,in mg,of C22H28FNa2O8Pin the portion of Dexamethasone Sodium Phosphate taken by the formula:
(516.41/472.45)C(rU/rS),
in which 516.41and 472.45are the molecular weights of dexamethasone sodium phosphate and dexamethasone phosphate,respectively;Cis the concentration,in mg per mL,of USP Dexamethasone Phosphate RSin the Standard preparation;and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 592
Phone Number:1-301-816-8139