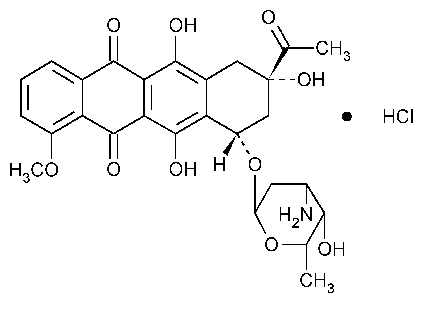
Daunorubicin Hydrochloride
5,12-Naphthacenedione,8-acetyl-10-[(3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-1-methoxy-,(8S-cis)-,hydrochloride.
(1S,3S)-3-Acetyl-1,2,3,4,6,11-hexahydro-3,5,12-trihydroxy-10-methoxy-6,11-dioxo-1-naphthacenyl 3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside hydrochloride [23541-50-6].
»Daunorubicin Hydrochloride has a potency equivalent to not less than 842µg and not more than 1030µg of C27H29NO10per mg.
Caution—Great care should be taken to prevent inhaling particles of Daunorubicin Hydrochloride and exposing the skin to it.
Packaging and storage—
Preserve in tight containers,protected from light and excessive heat.
Identification—
A:
The IRabsorption spectrum of a potassium bromide dispersion of it exhibits maxima only at the same wavelengths as that of a similar preparation of USP Daunorubicin Hydrochloride RS.
B:
The retention time of the main peak obtained with the Assay preparationcorresponds to that obtained with the Standard preparationas directed in the Assay.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 4.5and 6.5,in a solution containing 5mg per mL.
Water,Method Iá921ñ:
not more than 3.0%.
Assay—
Mobile phase—
Mix 62volumes of water and 38volumes of acetonitrile,and adjust with phosphoric acid to a pHof 2.2±0.2.The acetonitrile concentration may be varied to meet system suitability requirements and to provide a suitable elution time for daunorubicin.Filter the solution through a membrane filter (1µm or finer porosity),and degas.
Standard preparation—
Dissolve an accurately weighed quantity of USP Daunorubicin Hydrochloride RSin Mobile phaseto obtain a solution having a known concentration of about 250µg of daunorubicin per mL.
Resolution solution—
Prepare a solution of doxorubicin hydrochloride in the Standard preparationcontaining about 250µg per mL.
Assay preparation—
Transfer about 25mg of Daunorubicin Hydrochloride,accurately weighed,to a 100-mLvolumetric flask,dissolve in and dilute with Mobile phaseto volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The chromatograph is equipped with a 254-nm detector and a 4.6-mm ×30-cm column that contains packing L1.The flow rate is about 1.5mLper minute.Chromatograph the Resolution solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.7for doxorubicin and 1.0for daunorubicin;and the resolution,R,between the doxorubicin peak and the daunorubicin peak is not less than 3.Chromatograph replicate injections of the Standard preparation,and record the peak responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 5µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the potency,in µg of C27H29NO10per mg,taken by the formula:
100(C/W)(rU/rS),
in which Cis the concentration,in µg per mL,of daunorubicin in the Standard preparation;Wis the weight,in mg,of Daunorubicin Hydrochloride taken;and rUand rSare the daunorubicin peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 573
Phone Number:1-301-816-8335