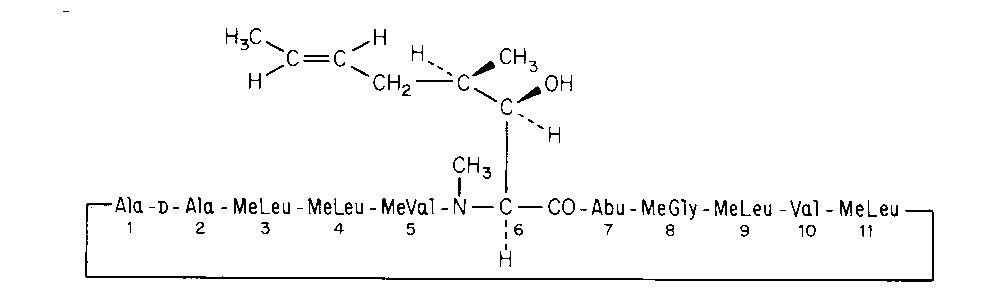
Cyclosporine
Cyclo[[(E)-(2S,3R,4R)-3-hydroxy-4-methyl-2-(methylamino)-6-octenoyl]-L-2-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl-L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl].
[R-[R*,R*-(E)]]-Cyclic(L-alanyl-D-alanyl-N-methyl-L-leucyl-N-methyl-L-leucyl-N-methyl-L-valyl-3-hydroxy-N,4-dimethyl-L-2-amino-6-octenoyl-L-a-aminobutyryl-N-methylglycyl-N-methyl-L-leucyl-L-valyl-N-methyl-L-leucyl) [59865-13-3].
»Cyclosporine contains not less than 98.5percent and not more than 101.5percent of cyclosporine A(C62H111N11O12),calculated on the dried basis.
Packaging and storage—
Preserve in tight,light-resistant containers.
Identification—
The chromatogram of the Assay preparationobtained as directed in the Assayexhibits a major peak for cyclosporine,the retention time of which corresponds to that exhibited in the chromatogram of the Standard preparation,as obtained in the Assay.
Loss on drying á731ñ—
Dry about 100mg,accurately weighed,in a capillary-stoppered bottle in vacuum at a pressure not exceeding 5mm of mercury at 60 for 3hours:it loses not more than 2.0%of its weight.
for 3hours:it loses not more than 2.0%of its weight.
Heavy metals,Method IIá231ñ:
0.002%.
Related compounds—
Using the chromatograms obtained from Standard preparation 2and the Assay preparationin the Assay,calculate the percentage of each impurity by the formula:
2000(C/W)(ri/rS2),
in which Cis the concentration,in mg per mL,of USP Cyclosporine RSin Standard preparation 2;Wis the weight,in mg,of Cyclosporine taken to prepare the Assay preparation;riis the response of an individual impurity observed in the chromatogram of the Assay preparation;and rS2is the response of the main cyclosporine peak in the chromatogram obtained from Standard preparation 2:not more than 0.7%of any individual impurity is found,and the sum of all such impurities is not more than 1.5%,any impurities corresponding to less than 0.05%being disregarded.
Assay—
Mobile phase—
Prepare a mixture of water,acetonitrile,tert-butyl methyl ether,and phosphoric acid (520:430:50:1).Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Diluent—
Prepare a mixture of acetonitrile and water (1:1).
Standard preparation 1—
Dissolve an accurately weighed quantity of USP Cyclosporine RSin Diluentto obtain a solution having a known concentration of about 1.25mg per mL.
Standard preparation 2—
Transfer 2.0mLof Standard preparation 1to a 250-mLvolumetric flask,dilute with Diluentto volume,and mix.This solution contains about 0.01mg of USP Cyclosporine RSper mL.
Assay preparation—
Dissolve about 25mg of Cyclosporine,accurately weighed,in Diluent,dilute with Diluentto 20.0mL,and mix.
Resolution solution—
Prepare a solution of USP Cyclosporine Resolution Mixture RSin Diluenthaving a concentration of about 1.25mg per mL.
Chromatographic system(see Chromatography á621ñ)—
The liquid chromatograph is equipped with a 210-nm detector,a 0.25-mm ×1-m stainless steel tube connected to a 4-mm ×25-cm column that contains 3-to 5-µm packing L1.The tube and column are maintained at 80 .The flow rate is about 1.2mLper minute.Chromatograph the Resolution solution,and record the responses as directed for Procedure:the cyclosporine Upeak and the main cyclosporine peak are resolved from each other.Chromatograph Standard preparation 1,and record the responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 1.0%.Chromatograph Standard preparation 2,and record the responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 10%.
.The flow rate is about 1.2mLper minute.Chromatograph the Resolution solution,and record the responses as directed for Procedure:the cyclosporine Upeak and the main cyclosporine peak are resolved from each other.Chromatograph Standard preparation 1,and record the responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 1.0%.Chromatograph Standard preparation 2,and record the responses as directed for Procedure:the relative standard deviation for replicate injections is not more than 10%.
Procedure—
[NOTE—Use peak areas where peak responses are indicated.]Separately inject equal volumes (about 20µL)of Standard preparation 1,Standard preparation 2,and the Assay preparationinto the chromatograph,record the chromatograms,and measure the peak responses.Calculate the percentage of cyclosporine A(C62H111N11O12)in the Cyclosporine taken by the formula:
(CP/10U)(rU/rS),
in which Cis the concentration,in mg per mL,of USP Cyclosporine RSin Standard preparation 1;Pis the specified purity,in µg per mg,of USP Cyclosporine RS;Uis the concentration,in mg per mL,of specimen in the Assay preparation;and rUand rSare the main cyclosporine peak responses obtained from the Assay preparationand Standard preparation 1,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 561
Pharmacopeial Forum:Volume No.27(2)Page 2135
Phone Number:1-301-816-8335