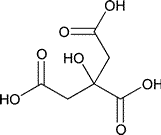
Add the following:
»Anhydrous Citric Acid contains not less than 99.5percent and not more than 100.5percent of C6H8O7,calculated on the anhydrous basis.
Labeling—
Where it is intended for use in dialysis solutions,it is so labeled.Where Anhydrous Citric Acid must be subjected to further processing during the preparation of injectable dosage forms to ensure acceptable levels of bacterial endotoxins,it is so labeled.Where Anhydrous Citric Acid is sterile,it is so labeled.
Clarity of solution—
[NOTE—The Test solutionis to be compared to Reference suspension Ain diffused daylight 5minutes after preparation of Reference suspension A.]
Hydrazine sulfate solution—
Transfer 1.0g of hydrazine sulfate to a 100-mLvolumetric flask,dissolve in and dilute with water to volume,and mix.Allow to stand for 4to 6hours before use.
Methenamine solution—
Transfer 2.5g of Methenamine to a 100-mLglass-stoppered flask,add 25.0mLof water,insert the glass stopper,and mix to dissolve.
Primary opalescent suspension—
[NOTE—This suspension is stable for 2months,provided it is stored in a glass container free from surface defects.The suspension must not adhere to the glass and must be well mixed before use.]Transfer 25.0mLof Hydrazine sulfate solutionto the Methenamine solutionin the 100-mLglass-stoppered flask.Mix,and allow to stand for 24hours.
Opalescence standard—
[NOTE—This suspension should not be used beyond 24hours after preparation.]Transfer 15.0mLof the Primary opalescent suspensionto a 1000-mLvolumetric flask,dilute with water to volume,and mix.
Reference suspensions—
Transfer 5.0mLof the Opalescence standardto a 100-mLvolumetric flask,dilute with water to volume,and mix to obtain Reference suspension A.Transfer 10.0mLof the Opalescence standardto a second 100-mLvolumetric flask,dilute with water to volume,and mix to obtain Reference suspension B.
Test solution—
Dissolve 2.0g of Anhydrous Citric Acid in about 5mLof water,dilute with water to 10mL,and mix.
Procedure—
Transfer a sufficient portion of the Test solutionto a test tube of colorless,transparent,neutral glass with a flat base and an internal diameter of 15to 25mm to obtain a depth of 40mm.Similarly transfer portions of Reference suspension A,Reference suspension B,and water to separate matching test tubes.Compare the Test solution,Reference suspension A,Reference suspension B,and water in diffused daylight,viewing vertically against a black background (see Visual Comparisonunder Spectrophotometry and Light-Scattering á851ñ).[NOTE—The diffusion of light must be such that Reference suspension Acan readily be distinguished from water,and that Reference suspension Bcan readily be distinguished from Reference suspension A.]The Test solutionshows the same clarity as that of water.
Color of solution—
Standard stock solutions—
Prepare three solutions,A,B,and C,containing,respectively,the following parts of ferric chloride CS,cobaltous chloride CS,cupric sulfate CS,and dilute hydrochloric acid (10g per L):
A—
2.4:0.6:0:7.0.
B—
2.4:1.0:0.4:6.2.
C—
9.6:0.2:0.2:0.
Standard solutions—
[NOTE—Prepare the Standard solutionsimmediately before use.]Transfer 2.5mLof Standard stock solution Ato a 100-mLvolumetric flask,dilute with dilute hydrochloric acid (10g per L)to volume,and mix to obtain Standard solution A.Transfer 2.5mLof Standard stock solution Bto a 100-mLvolumetric flask,dilute with dilute hydrochloric acid (10g per L)to volume,and mix to obtain Standard solution B.Transfer 0.75mLof Standard stock solution Cto a 100-mLvolumetric flask,dilute with dilute hydrochloric acid (10g per L)to volume,and mix to obtain Standard solution C.
Test solution—
Use the Test solutionprepared as directed in the test for Clarity of solution.
Procedure—
Transfer a sufficient portion of the Test solutionto a test tube of colorless,transparent,neutral glass with a flat base and an internal diameter of 15to 25mm to obtain a depth of 40mm.Similarly transfer portions of Standard solution A,Standard solution B,Standard solution C,and water to separate matching test tubes.Compare the Test solution,Standard solution A,Standard solution B,Standard solution C,and water in diffused daylight,viewing vertically against a white background (see Visual Comparisonunder Spectrophotometry and Light-Scattering á851ñ).The Test solutionis not more intensely colored than Standard solutions A,B,Cor water.
Identification,Infrared Absorption á197Kñ—
Dry the substance to be examined at 105 for 2hours.
for 2hours.
Bacterial endotoxins á85ñ—
The level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s)in which Anhydrous Citric Acid is used can be met.Where the label states that Anhydrous Citric Acid must be subjected to further processing during the preparation of injectable dosage forms,the level of bacterial endotoxins is such that the requirement in the relevant dosage form monograph(s)in which Anhydrous Citric Acid is used can be met.
Sterility á71ñ—
Where the label states that Anhydrous Citric Acid is sterile,it meets the requirements for Sterility á71ñin the relevant dosage form monograph(s)in which Anhydrous Citric Acid is used.
Water,Method Iá921ñ:
not more than 1.0%.
Residue on ignition á281ñ:
not more than 0.1%,determined on 1.0g.
Readily carbonizable substances—
Transfer 1.0g of powdered Anhydrous Citric Acid to a 22-×175-mm test tube previously rinsed with 10mLof sulfuric acid TSand allowed to drain for 10minutes.Add 10mLof sulfuric acid TS,agitate until solution is complete,and immerse in a water bath at 90±1ºfor 60±0.5minutes,keeping the level of the acid below the level of the water during the entire period.Cool the tube in running water,and transfer the acid to a color-comparison tube:the color of the acid is not darker than that of a similar volume of Matching Fluid K(see Color and Achromicity á631ñ)in a matching tube,the tubes being observed vertically against a white background.
Sulfate—
Standard sulfate solution A—
To 181mg of dibasic potassium sulfate in a 100-mLvolumetric flask,add a few mLof 30%alcohol,swirl to dissolve,dilute with 30%alcohol to volume,and mix.Immediately before use,transfer 10.0mLof this solution to a 1000-mLvolumetric flask,dilute with 30%alcohol to volume,and mix.This solution contains 10µg of sulfate per mL.
Standard sulfate solution B—
To 181mg of dibasic potassium sulfate in a 100-mLvolumetric flask,add a few mLof water,swirl to dissolve,dilute with water to volume,and mix.Immediately before use,transfer 10.0mLof this solution to a 1000-mLvolumetric flask,dilute with water to volume,and mix.This solution contains 10µg of sulfate per mL.
Citric acid solution—
Dissolve 2.0g of Anhydrous Citric Acid in about 10mLof water,dilute with water to 30mL,and mix.
Procedure—
To 4.5mLof Standard sulfate solution Aadd 3mLof a barium chloride solution (1in 4),shake,and allow to stand for 1minute.To 2.5mLof the resulting suspension,add 15mLof the Citric acid solutionand 0.5mLof 5Nacetic acid,and mix (test solution).Prepare the Standard solution in the same manner,except use 15mLof Standard sulfate solution Binstead of the Citric acid solution:any turbidity produced in the test solution after 5minutes standing is not greater than that produced in the Standard solution (0.015%).
Heavy metals á231ñ:
0.001%.
Limit of oxalic acid—
Prepare a citric acid solution by dissolving 800mg of Anhydrous Citric Acid in 4mLof water.Add 3mLof hydrochloric acid and 1g of granular zinc,boil for 1minute,and allow to stand for 2minutes.Transfer the supernatant to a test tube containing 0.25mLof a phenylhydrazine hydrochloride solution (1in 100),and heat to boiling.Cool rapidly,transfer to a graduated cylinder,and add an equal volume of hydrochloric acid and 0.25mLof a potassium ferricyanide solution (1in 20).Shake,and allow to stand for 30minutes (test solution).Concomitantly prepare a control solution in the same manner,except use 4mLof an oxalic acid solution containing 0.10mg per mL,equivalent to 0.0714mg of anhydrous oxalic acid per mL,instead of the citric acid solution:any pink color produced in the test solution is not more intense than that produced in the control solution (0.036%).
Limit of aluminum(where it is labeled as intended for use in dialysis)—
Standard aluminum solution—
To 352mg of aluminum potassium sulfate in a 100-mLvolumetric flask,add a few mLof water,swirl to dissolve,add 10mLof diluted sulfuric acid,dilute with water to volume,and mix.Immediately before use,transfer 1.0mLof this solution to a 100-mLvolumetric flask,dilute with water to volume,and mix.
pH6.0Acetate buffer—
Dissolve 50g of ammonium acetate in 150mLof water,adjust with glacial acetic acid to a pHof 6.0,dilute with water to 250mL,and mix.
Test solution—
Dissolve 20.0g of Anhydrous Citric Acid in 100mLof water,and add 10mLof pH6.0Acetate buffer.Extract this solution with successive portions of 20,20,and 10mLof a 0.5%solution of 8-hydroxyquinoline in chloroform,combining the chloroform extracts in a 50-mLvolumetric flask.Dilute the combined extracts with chloroform to volume,and mix.
Standard solution—
Prepare a mixture of 2.0mLof Standard aluminum solution,10mLof pH6.0Acetate buffer,and 98mLof water.Extract this mixture as described for the Test solution,dilute the combined extracts with chloroform to volume,and mix.
Blank solution—
Prepare a mixture of 10mLof pH6.0Acetate bufferand 100mLof water.Extract this mixture as described for the Test solution,dilute the combined extracts with chloroform to volume,and mix.
Procedure—
Determine the fluorescence intensities of the Test solutionand the Standard solutionin a fluorometer set at an excitation wavelength of 392nm and an emission wavelength of 518nm,using theBlank solutionto set the instrument to zero.The fluorescence of the Test solutiondoes not exceed that of the Standard solution(0.2µg per g).
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
Place about 0.550g of Anhydrous Citric Acid in a tared flask,and weigh accurately.Dissolve in 50mLof water,add 0.5mLof phenolphthalein TS,and titrate with 1Nsodium hydroxide VS.Each mLof 1Nsodium hydroxide is equivalent to 64.03mg of C6H8O7. USP28
USP28
Auxiliary Information—
Staff Liaison:Justin Lane,B.S.,Scientific Associate
Expert Committee:(EMC)Excipients:Monograph Content
USP28–NF23Page 483
Pharmacopeial Forum:Volume No.30(5)Page 1851
Phone Number:1-301-816-8323