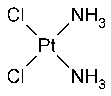
Cisplatin
»Cisplatin contains not less than 98.0percent and not more than 102.0percent of Cl2H6N2Pt,calculated on the anhydrous basis.
Caution—Cisplatin is potentially cytotoxic.Great care should be taken to prevent inhaling particles and exposing the skin to it.
Packaging and storage—
Preserve in tight containers.Protect from light.
USP Reference standards á11ñ—
USP Cisplatin RS.USP Transplatin RS.USP Potassium Trichloroammineplatinate RS.
Identification—
A:
The retention time of the major peak in the chromatogram of the Assay preparationcorresponds to that of the Standard preparationas obtained in the Assay.
B:
Infrared Absorption á197Kñ.
C:
Spray reagent—Add 5.6g of stannous chloride to 10mLof hydrochloric acid,and stir for 5minutes.[NOTE—It is not necessary that all of the solids dissolve.]Dissolve 0.2g of potassium iodide in 90mLof water.Mix the two solutions together.Disregard any precipitate that is formed.Store in the dark.The solution is usable for at least 1week.
Procedure—
Prepare a test solution containing 1mg of Cisplatin per mLand a Standard solution containing 1mg of USP Cisplatin RSper mL,both in dimethylformamide.Apply separately 5-µLquantities of each solution to a thin-layer chromatographic plate coated with a 0.25-mm layer of chromatographic silica gel mixture (see Chromatography á621ñ).Place the plate in a suitable chromatographic chamber containing a filter paper lining and equilibrated for 30minutes with a developer consisting of a mixture of acetone and 1Nnitric acid (180:20).Develop the plate for a distance of about 8cm from the origin.Remove the plate,and allow it to air-dry.Complete the drying by heating in a forced-air oven at about 100 for 1minute.Spray the plate with Spray reagent,heat it in an oven at about 100
for 1minute.Spray the plate with Spray reagent,heat it in an oven at about 100 for 5minutes,cool,and spray with a 1in 50solution of potassium iodide in water,to bring out the full color of the spots:the principal spot from the test solution corresponds in appearance and RFvalue to that produced by the Standard solution.
for 5minutes,cool,and spray with a 1in 50solution of potassium iodide in water,to bring out the full color of the spots:the principal spot from the test solution corresponds in appearance and RFvalue to that produced by the Standard solution.
Crystallinity á695ñ:
meets the requirements.
Water,Method Iá921ñ:
not more than 1.0%.
UVpurity ratio—
[NOTE—Cleanse all glassware with a mixture of hydrochloric acid and nitric acid (3:1),rinse thoroughly with water,and dry before use.Do not use dichromate for cleaning.Do not use acetone or pressurized air for drying.Protect the test solution from light,and use within 1hour after its preparation.]Transfer 98.5±0.5mg of ground Cisplatin to a 100-mLvolumetric flask,and add 0.1Nhydrochloric acid to volume.Using a clean magnetic stir bar,alternately stir at a high speed for 5minutes and sonicate for 10seconds until complete solution is effected,inverting the flask frequently to remove particles that may cling to the neck.Obtain the UVabsorption spectrum,using thoroughly rinsed 2-cm cells,with 0.1Nhydrochloric acid in the reference cell:the ratio of the absorbance at the maximum near 301nm to that at the minimum near 246nm is not less than 4.5.
Limit of trichloroammineplatinate—
Mobile phase—
Transfer 0.8g of ammonium sulfate to a 2-liter volumetric flask,dissolve in water,and dilute with water to volume.Degas,and filter through a membrane filter prior to use.The pHof this solution is 5.9±0.1.Make adjustments to the ionic strength of the Mobile phase,if necessary,to meet the system suitability requirements.
Standard preparation—
[NOTE—Use low-actinic glassware.]Dissolve a suitable quantity of USP Potassium Trichloroammineplatinate RS,accurately weighed,in saline TS,and dilute quantitatively with saline TSto obtain a solution having a known concentration of about 6µg per mL.Use within 4hours.
Test preparation—
[NOTE—Use low-actinic glassware.]Transfer about 50mg of Cisplatin,accurately weighed,to a 100-mLvolumetric flask,and dilute with saline TSto volume.Completely dissolve by stirring by mechanical means for 30minutes.Use within 4hours.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 209-nm detector and a 4.6-mm ×25-cm column that contains packing L14.The flow rate is about 2mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed under Procedure:the resolution,R,between the saline TSpeak and the trichloroammineplatinate peak is not less than 2.0,and the relative standard deviation for replicate injections is not more than 3.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Test preparationinto the chromatograph,record the chromatograms,and measure the areas for the peaks due to trichloroammineplatinate.The relative retention times are about 1.0for cisplatin (in the void volume)and 5.0for trichloroammineplatinate.Calculate the percentage of trichloroammineplatinate taken by the formula:
10(318.48/357.58)(rU/rS)(C/W),
in which 318.48and 357.58are the formula weights of trichloroammineplatinate and potassium trichloroammineplatinate,respectively,rUand rSare the peak areas obtained from the Test preparationand the Standard preparation,respectively,Cis the concentration,in µg per mL,of the Standard preparation,and Wis the weight,in mg,of Cisplatin taken:not more than 1.0%is found.
Limit of transplatin—
Mobile phase—
Prepare a 0.18Msolution in water of monobasic potassium phosphate.Adjust with phosphoric acid to a pHof 3.2,and filter.
Stock standard solution—
Transfer about 10mg of USP Transplatin RS,accurately weighed,to a 200-mLvolumetric flask,dilute with saline TSto volume,and dissolve by stirring by mechanical means for 30minutes.
Working standard solution—
Pipet 5mLof Stock standard solution into a 25-mLvolumetric flask containing about 12mg of USP Cisplatin RS.Dilute with saline TSto volume,and stir by mechanical means for 30minutes to dissolve.
Standard preparation—
Pipet 10mLof Working standard solutioninto a 50-mLvolumetric flask.Add 5.0mLof a 1in 200solution of thiourea,prepared fresh daily,and 5.0mLof 1Nhydrochloric acid.Dilute with saline TSto volume,and mix.Place about 10mLof this solution in a suitable serum vial,seal with a polytef-lined closure,and heat in a heating block at 60±0.5 for 60minutes.Remove,and cool to room temperature.
for 60minutes.Remove,and cool to room temperature.
Test solution—
Transfer about 50mg of Cisplatin,accurately weighed,to a 100-mLvolumetric flask,dilute with saline TSto volume,and dissolve by stirring by mechanical means for 30minutes.
Test preparation—
Pipet 10mLof Test solutioninto a 50-mLvolumetric flask,and proceed as directed for Standard preparation,beginning with “add 5.0mLof a 1in 200solution of thiourea.”
Resolution solution—
Place about 10mg of USP Cisplatin RSin a 200-mLvolumetric flask,dilute with saline TSto volume,and stir by mechanical means for 30minutes to dissolve.Pipet 10mLof this solution and 10mLof Stock standard solutioninto a 50-mLvolumetric flask,and proceed as directed for Standard preparation,beginning with “add 5.0mLof a 1in 200solution of thiourea.”
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.6-mm ×25-cm column that contains packing L9.The column is maintained throughout at a temperature of 45 .The flow rate is about 2.0mLper minute.Condition the column by pumping Mobile phaseat a flow rate of 2.0mLper minute for 30minutes,then at 0.5mLper minute for 30minutes,and then again at 2.0mLper minute for 30minutes.Chromatograph the Standard preparation.The retention time of the derivatized transplatin is between 5.0and 9.0minutes;or,if it is not,modify the Mobile phaseas necessary,and recondition the column.The column efficiency,n,is not less than 2500.Chromatograph the Resolution solution.The resolution,R,is not less than 1.7.Chromatograph the Standard preparationas directed for Procedure:the relative standard deviation for replicate injections is not more than 4.0%.
.The flow rate is about 2.0mLper minute.Condition the column by pumping Mobile phaseat a flow rate of 2.0mLper minute for 30minutes,then at 0.5mLper minute for 30minutes,and then again at 2.0mLper minute for 30minutes.Chromatograph the Standard preparation.The retention time of the derivatized transplatin is between 5.0and 9.0minutes;or,if it is not,modify the Mobile phaseas necessary,and recondition the column.The column efficiency,n,is not less than 2500.Chromatograph the Resolution solution.The resolution,R,is not less than 1.7.Chromatograph the Standard preparationas directed for Procedure:the relative standard deviation for replicate injections is not more than 4.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Test preparationand the Standard preparationinto the chromatograph,record the chromatograms,and measure the areas of the transplatin peaks.The relative retention times are about 1.0for cisplatin and 1.3for transplatin.Calculate the percentage of transplatin taken by the formula:
10(C/W)(rU/rS),
in which Cis the concentration,in µg per mL,of USP Transplatin RSin the Working standard solution,Wis the weight,in mg,of Cisplatin taken to prepare the Test solution,and rUand rSare the peak areas obtained from the Test preparationand the Standard preparation,respectively.Not more than 2.0%is found.
Platinum content—
[NOTE—Thoroughly cleanse all glassware with nitric acid,and rinse with Purified Water,to prevent “mirroring”of the platinum precipitate.]Transfer about 0.5g of Cisplatin,accurately weighed,to a 600-mLbeaker.Add 300mLof 0.1Nhydrochloric acid,and slowly dissolve by heating nearly to boiling on a hot plate covered with an insulating pad,and stirring frequently with a glass stirring rod.When solution is complete,remove the insulating pad,and boil for about 10minutes.Remove the beaker from the hot plate,allow to cool for 1minute without stirring,and filter through quantitative,fine-porosity,smooth,dense,ashless filter paper,collecting the filtrate in a 600-mLbeaker,completing the transfer to the filter with hot water.Wash the filter with hot water.Place the beaker containing the combined filtrate and washings on a hot plate,and evaporate to a volume of about 300mL.Place a glass stirring rod in the beaker,and heat the solution to boiling.Slowly add to the center of the beaker,by dropwise additions,10.0mLof hydrazine hydrate,85%.[Caution—Hydrazine is toxic.
]Add 2drops of 10Nsodium hydroxide,boil for 10minutes to coagulate the precipitate for ease of filtration,cool,and filter through quantitative,medium-porosity,smooth,ashless filter paper.Rinse the beaker with hot water,and pour the rinsings onto the filter.Wipe the beaker and the stirring rod with small pieces of the same kind of paper used for this filtration,and place these and the filter containing the precipitate in a No.1porcelain crucible,previously ignited to constant weight.Dry on a hot plate covered with an insulating pad,slowly increase the heat to char,and ignite for 1hour at 800 .Cool in a desiccator,and again weigh:the weight of the platinum so obtained is between 64.42%and 65.22%of the weight of Cisplatin taken,on the anhydrous basis.
.Cool in a desiccator,and again weigh:the weight of the platinum so obtained is between 64.42%and 65.22%of the weight of Cisplatin taken,on the anhydrous basis.
Assay—
Mobile phase—
Prepare a suitable solution by mixing ethyl acetate,methanol,dimethylformamide,and degassed water (25:16:5:5),and degas.
Standard preparation—
Dissolve an accurately weighed quantity of USP Cisplatin RSquantitatively in dimethylformamide to obtain a solution having a known concentration of about 1mg per mL.Use within 1hour.
Assay preparation—
Dissolve about 100mg of Cisplatin,accurately weighed,in dimethylformamide in a 100-mLvolumetric flask,dilute with dimethylformamide to volume,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 310-nm detector and a 4.0-mm ×30-cm column that contains packing L8.The flow rate is about 2.0mLper minute.Chromatograph the Standard preparation,and record the peak response as directed under Procedure:the relative standard deviation for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 40µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in mg,of Cl2H6N2Pt in the portion of Cisplatin taken by the formula:
100C(rU/rS),
in which Cis the concentration,in mg per mL,of USP Cisplatin RSin the Standard preparation,and rUand rSare the peak responses obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(PA6)Pharmaceutical Analysis 6
USP28–NF23Page 480
Phone Number:1-301-816-8389