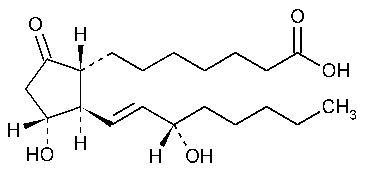
Alprostadil
»Alprostadil contains not less than 95.0percent and not more than 105.0percent of C20H34O5,calculated on the anhydrous basis.
Caution—Great care should be taken to prevent inhaling particles of Alprostadil and exposing the skin to it.
Packaging and storage—
Preserve in tight containers,and store in a refrigerator.
Identification,
Infrared Absorption á197Mñ.
Water,Method Iá921ñ:
not more than 0.5%,using 0.5g.
Residue on ignition á281ñ:
not more than 0.5%,using 0.3g.
Limit of chromium—
Standard solution—
Dissolve an accurately weighed quantity of chromium trichloride in 0.05Mnitric acid,and dilute,stepwise if necessary,with 0.05Mnitric acid to obtain a solution having a known concentration of about 3.04µg per mL.Transfer 2mLof this solution to a 100-mLvolumetric flask,dilute with alcohol to volume,and mix.This solution contains 20ng of chromium per mL.
Test solution—
Transfer about 10mg of Alprostadil,accurately weighed,to a 10-mLvolumetric flask,dissolve in and dilute with alcohol to volume,and mix.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto a suitable graphite furnace atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering á851ñ),equipped with a chromium hollow-cathode lamp,with a drying temperature of 100 ,an ashing temperature of 1000
,an ashing temperature of 1000 ,and an atomization temperature of 2700
,and an atomization temperature of 2700 .Use alcohol as the blank.Concomitantly determine the absorbances at the chromium emission line at 357.9nm,and calculate the percentage of chromium (Cr)in the portion of Alprostadil taken by the formula:
.Use alcohol as the blank.Concomitantly determine the absorbances at the chromium emission line at 357.9nm,and calculate the percentage of chromium (Cr)in the portion of Alprostadil taken by the formula:
100(CS/CA)(AU/AS),
in which CSis the concentration,in mg per mL,of chromium in the Standard solution;CAis the concentration,in mg per mL,of Alprostadil in the Test solution;and AUand ASare the absorbances obtained from the Test solutionand Standard solution,respectively:not more than 0.002%is found.
Limit of rhodium—
Standard solution—
Dissolve an accurately weighed quantity of rhodium chloride hydrate in 1.2Mhydrochloric acid,and dilute,stepwise if necessary,with 1.2Mhydrochloric acid to obtain a solution having a known concentration of 100µg of rhodium per mL.Transfer 5mLof this solution to a 100-mLvolumetric flask,dilute with alcohol to volume,and mix.Transfer 2mLof this solution to a 200-mLvolumetric flask,dilute with alcohol to volume,and mix.This solution contains 50ng of rhodium per mL.
Test solution—
Transfer about 20mg of Alprostadil,acccurately weighed,to a 10-mLvolumetric flask,dissolve in and dilute with alcohol to volume,and mix.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto a suitable graphite furnace atomic absorption spectrophotometer (see Spectrophotometry and Light-Scattering á851ñ),equipped with a rhodium hollow-cathode lamp,with a drying temperature of 100 ,an ashing temperature of 1000
,an ashing temperature of 1000 ,and an atomization temperature of 2800
,and an atomization temperature of 2800 .Use alcohol as the blank.Concomitantly determine the absorbances at the rhodium emission line at 343.5nm,and calculate the percentage of rhodium (Rh)in the portion of Alprostadil taken by the formula:
.Use alcohol as the blank.Concomitantly determine the absorbances at the rhodium emission line at 343.5nm,and calculate the percentage of rhodium (Rh)in the portion of Alprostadil taken by the formula:
100(CS/CA)(AU/AS),
in which CSis the concentration,in mg per mL,of rhodium in the Standard solution;CAis the concentration,in mg per mL,of Alprostadil in the Test solution;and AUand ASare the absorbances obtained from the Test solutionand the Standard solution,respectively:not more than 0.002%is found.
Limit of foreign prostaglandins—
TEST1—
NOTE—Use freshly prepared solutions.Measure the peak responses at the following wavelengths:prostaglandin A1at 224nm;prostaglandin B1at 280nm;and all other foreign prostaglandin impurities at 200nm.
Mobile phase—
Proceed as directed in the Assay.
Standard solution—
Dissolve accurately weighed quantities of USP Alprostadil RS,USP Prostaglandin A1RS,and USP Prostaglandin B1RSin a mixture of methanol and water (9:1),and dilute quantitatively,and stepwise if necessary,with a mixture of methanol and water (9:1)to obtain a solution having known concentrations of about 6µg per mL,15µg per mL,and 6µg per mL,respectively.
Test solution—
Dissolve about 15mg of Alprostadil,accurately weighed,in 5mLof a mixture of methanol and water (9:1),and mix.
Chromatographic system—
Proceed as directed in the Assay.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the resolution between prostaglandin A1and alprostadil is not less than 7.5,and the relative standard deviation from the peaks at their respective wavelength for replicate injections is not more than 4.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the peak responses at 200nm,224nm,and 280nm.Calculate the percentage of prostaglandin A1and prostaglandin B1in the portion of Alprostadil taken by the formula:
500(CS/W)(rI/rS),
in which CSis the concentration,in mg per mL,of USP Prostaglandin A1RSor USP Prostaglandin B1RSin the Standard solution;Wis the weight,in mg,of Alprostadil taken for the Test solution;riis the peak response for prostaglandin A1or prostaglandin B1obtained from the Test solution;and rSis the peak response of prostaglandin A1or prostaglandin B1obtained from the Standard solution:not more than 1.5%of prostaglandin A1is found;and not more than 0.1%of prostaglandin B1is found.Calculate the percentage of each impurity occurring at 200nm and eluting before prostaglandin A1in the portion of Alprostadil taken by the formula:
500(CS/W)(rI/rS),
in which CSis the concentration,in mg per mL,of USP Alprostadil RSin the Standard solution;riis the peak response for each impurity obtained from the Test solution;rSis the peak response for alprostadil obtained from the Standard solution;and the other terms are as defined herein:not more than 0.9%of any foreign prostaglandin impurity is found.Calculate the percentage of any impurity having a relative retention time of 0.6,relative to the prostaglandin A1peak detected at 224nm,in the portion of Alprostadil taken by the formula:
500(CS/W)(rI/rS),
in which CSis the concentration,in mg per mL,of USP Prostaglandin A1RSin the Standard solution;rIis the peak response for any impurity having a relative retention time of 0.6,relative to the prostaglandin A1peak,obtained from the Test solution;rSis the peak response of prostaglandin A1obtained from the Standard solution;and the other terms are as defined herein:not more than 0.9%of any impurity having a relative retention time of 0.6,relative to the prostaglandin A1peak,is found.
TEST2—
Mobile phase—
Prepare a filtered and degassed mixture of methanol,acetonitrile,and 0.02Mmonobasic potassium phosphate (2:1:1),and adjust with phosphoric acid to a pHof 3.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of USP Alprostadil RSin a mixture of acetonitrile and water (1:1)to obtain a solution having a known concentration of about 10µg per mL.
Test solution—
Dissolve about 25mg of Alprostadil,accurately weighed,in 5mLof a mixture of acetonitrile and water (1:1),using ultrasound if necessary.
Identification solution—
Use the Standard solutionunder Test 1.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a photodiode array detector or equivalent capable of detecting UVwavelengths between 200nm and 300nm and a 4.6-mm x 25-cm column that contains packing L1.The flow rate is about 1.2mLper minute.Chromatograph the Identification solutionas directed for Procedure:the relative retention times for prostaglandin A1and alprostadil are about 1.2and 1.0,respectively;the resolution between prostaglandin A1and alprostadil is not less than 4.0.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative standard deviation determined from the main peak for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the peak responses at 200nm,224nm,and 280nm.Calculate the percentage of each impurity occurring at 200nm and eluting after prostaglandin A1,excluding prostaglandin B1,in the portion of Alprostadil taken by the formula:
500(CS/W)(rI/rS),
in which CSis the concentration,in mg per mL,of USP Alprostadil RSin the Standard solution;Wis the weight,in mg,of Alprostadil taken for the Test solution;rIis the peak response for each impurity obtained from the Test solution;and rSis the peak response for alprostadil obtained from the Standard solution:the sum of the peaks having relative retention times of 2.0and 2.3is not more than 0.6%;not more than 0.9%of any other foreign prostaglandin impurity is found;and not more than 2.0%of total impurities is found,the results for Test 1and Test 2being added.
Organic volatile impurities,Method IVá467ñ:
meets the requirements.
Assay—
NOTE—Use freshly prepared solutions.
Mobile phase—
Prepare a filtered and degassed mixture of methanol,0.1Mmonobasic potassium phosphate and acetonitrile (2:2:1),and adjust with phosphoric acid to a pHof 3.0.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Internal standard solution—
Dissolve an accurately weighed quantity of ethylparaben in a mixture of methanol and water (9:1),and dilute quantitatively,and stepwise if necessary,with a mixture of methanol and water (9:1)to obtain a solution having a known concentration of about 0.05mg per mL.
Standard preparation—
Dissolve an accurately weighed quantity of USP Alprostadil RSin a mixture of methanol and water (9:1),and dilute quantitatively,and stepwise if necessary,with a mixture of methanol and water (9:1)to obtain a solution having a known concentration of about 0.3mg per mL.To 2.0mLof this solution,add 1.0mLof Internal standard solution,and mix.
System suitability solution—
Dilute an accurately measured amount of USP Prostaglandin A1RSwith Standard preparationto obtain a solution having a concentration of 4.5µg of prostaglandin A1per mL.To 2.0mLof this solution,add 1.0mLof Internal standard solution,and mix.
Assay preparation—
Transfer about 7.5mg of Alprostadil,accurately weighed,to a 25-mLvolumetric flask,dissolve in and dilute with a mixture of methanol and water (9:1)to volume,and mix.To 2.0mLof this solution,add 1.0mLof Internal standard solution,and mix.
Chromatographic system (see Chromatography á621ñ)—
The liquid chromatograph is equipped with a photodiode array detector or equivalent capable of detecting UVwavelengths between 200nm and 300nm and a 4.6-mm ×25-cm column that contains packing L1.The column temperature is maintained at 40 .The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the resolution,R,between prostaglandin A1and alprostadil is not less than 7.5,and between prostaglandin A1and ethylparaben is not less than 2.0;and the relative standard deviation determined from the peak area ratio of alprostadil to ethylparaben for replicate injections is not more than 2.0%.
.The flow rate is about 1mLper minute.Chromatograph the System suitability solution,and record the peak responses as directed for Procedure:the resolution,R,between prostaglandin A1and alprostadil is not less than 7.5,and between prostaglandin A1and ethylparaben is not less than 2.0;and the relative standard deviation determined from the peak area ratio of alprostadil to ethylparaben for replicate injections is not more than 2.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms at 200nm,and measure the areas for the major peaks.Calculate the quantity,in mg,of C20H34O5in the portion of Alprostadil taken by the formula:
25C(RU/RS),
in which Cis the concentration,in mg per mL,of USP Alprostadil RSin the Standard preparation;and RUand RSare the peak area ratios of alprostadil to ethylparaben obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:Clydewyn M.Anthony,Ph.D.,Scientist
Expert Committee:(PA1)Pharmaceutical Analysis 1
USP28–NF23Page 71
Pharmacopeial Forum:Volume No.29(5)Page 1412
Phone Number:1-301-816-8139