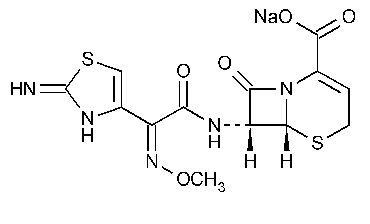
Ceftizoxime Sodium
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,7-[[(2,3-dihydro-2-imino-4-thiazoly)(methoxyimino)acetyl]amino]-8-oxomonosodium salt,[6R-[6a,7b(Z)]]-.
Sodium (6R,7R)-7-[2-(2-imino-4-thiazolin-4-yl)glyoxylamido]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate 72-(Z)-(O-methyloxime) [68401-82-1].
»Ceftizoxime Sodium contains the equivalent of not less than 850µg and not more than 995µg of ceftizoxime (C13H13N5O5S2)per mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
A:
The chromatogram of the Assay preparationobtained as directed in the Assayexhibits a major peak for ceftizoxime,the retention time of which corresponds to that exhibited in the chromatogram of the Standard preparationobtained as directed in the Assay.
B:
It responds to the tests for Sodium á191ñ.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 6.0and 8.0,in a solution (1in 10).
Water,Method Iá921ñ:
not more than 8.5%.
Other requirements—
Where the label states that Ceftizoxime Sodium is sterile,it meets the requirements for Sterilityand Bacterial endotoxinsunder Ceftizoxime for Injection.Where the label states that Ceftizoxime Sodium must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Bacterial endotoxinsunder Ceftizoxime for Injection.
Assay—
pH3.6buffer—
Dissolve 1.42g of citric acid monohydrate and 1.73g of dibasic sodium phosphate in water to obtain 1000mLof solution.
pH7.0buffer—
Dissolve 3.63g of monobasic potassium phosphate and 10.73g of dibasic sodium phosphate in water to obtain 1000mLof solution.
Mobile phase—
Prepare a mixture of pH3.6bufferand acetonitrile (about 9:1).Filter through a filter (1µm or finer porosity),and degas.Adjust the composition,if necessary,to meet the performance requirements under Chromatographic system.
Internal standard solution—
Dissolve 1.2g of salicylic acid in 10mLof methanol,and dilute with pH7.0bufferto obtain 200mLof solution.
Standard preparation—
Dissolve a suitable quantity of USP Ceftizoxime RS,accurately weighed,in pH7.0bufferto obtain a solution having a known concentration of about 1mg of ceftizoxime (C13H13N5O5S2)per mL.Transfer 2.0mLof this solution to a 100-mLvolumetric flask,add 5.0mLof Internal standard solution,dilute with pH7.0bufferto volume,and mix.This Standard preparationcontains about 0.02mg of ceftizoxime per mL.
Assay preparation—
Using a suitable quantity of Ceftizoxime Sodium,accurately weighed,proceed as directed under Standard preparation.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.0-mm ×30-cm column that contains 5-to 10-µm packing L1.The flow rate is about 2mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed under Procedure:the column efficiency determined from the analyte peak is not less than 2000theoretical plates,the tailing factor for the analyte peak is not more than 2,the resolution,R,between the analyte and internal standard peaks is not less than 4,and the relative standard deviation for replicate injections is not more than 2%.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.The relative retention times are about 0.6for ceftizoxime and 1.0for salicylic acid.Calculate the quantity,in µg,of ceftizoxime per mg of the Ceftizoxime Sodium taken by the formula:
1000(C/M)(RU/RS),
in which Cis the concentration,in mg of ceftizoxime (C13H13N5O5S2)per mL,of the Standard preparation;Mis the concentration,in mg per mL,of the Assay preparationbased on the weight of Ceftizoxime Sodium taken and the extent of dilution;and RUand RSare the peak response ratios of the ceftizoxime peak to the internal standard peak obtained from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 404
Phone Number:1-301-816-8335