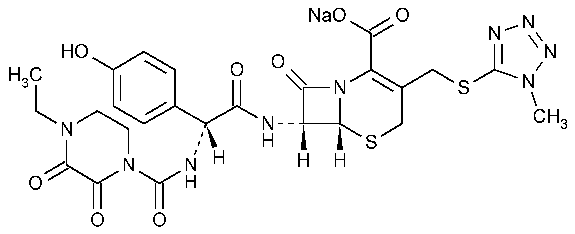
Cefoperazone Sodium
5-Thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid,7-[[[[(4-ethyl-2,3-dioxo-1-piperazinyl)carbonyl]amino](4-hydroxyphenyl)acetyl]amino]-3-[[(1-methyl-1H-tetrazol-5-yl)thio]methyl]-8-oxo-,monosodium salt,[6R-[6a,7b(R*)]]-.
Sodium (6R,7R)-7-[(R)-2-(4-ethyl-2,3-dioxo-1-piperazinecarboxamido)-2-(p-hydroxyphenyl)acetamido-3-[[(1-methyl-H-tetrazol-5-yl)thio]methyl]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate [62893-20-3].
»Cefoperazone Sodium contains the equivalent of not less than 870µg and not more than 1015µg of cefoperazone (C25H27N9O8S2)per mg,calculated on the anhydrous basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
Where it is intended for use in preparing injectable dosage forms,the label states that it is sterile or must be subjected to further processing during the preparation of injectable dosage forms.
Identification—
A:
The retention time of the major peak for cefoperazone in the chromatogram of the Assay preparationcorresponds to that in the chromatogram of the Standard preparation,as obtained in the Assay.
B:
It responds to the tests for Sodium á191ñ.
Crystallinity á695ñ:
meets the requirements.
pHá791ñ:
between 4.5and 6.5,in a solution (1in 4).
Water,Method Iá921ñ:
not more than 5.0%.
Other requirements—
Where the label states that Cefoperazone Sodium is sterile,it meets the requirements for Sterilityand Bacterial endotoxinsunder Cefoperazone for Injection.Where the label states that Cefoperazone Sodium must be subjected to further processing during the preparation of injectable dosage forms,it meets the requirements for Bacterial endotoxinsunder Cefoperazone for Injection.
Assay—
Mobile phase—
Place 14mLof triethylamine and 5.7mLof glacial acetic acid in a 100-mLvolumetric flask,dilute with water to volume,and mix.Prepare a suitable mixture of water,acetonitrile,1Nacetic acid,and this solution (876:120:2.8:1.2).Filter through a membrane filter (1-µm or finer porosity),and degas.
Standard preparation—
Dissolve a suitable quantity of USP Cefoperazone Dihydrate RS,accurately weighed,in Mobile phaseto obtain a solution having a known concentration of about 0.16mg of cefoperazone (C25H27N9O8S2)per mL.
Assay preparation—
Using a suitable quantity of Cefoperazone Sodium,accurately weighed,proceed as directed under Standard preparation.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 4.0-mm ×30-cm column that contains packing L1.The flow rate is about 2mLper minute.Chromatograph the Standard preparation,and record the peak responses as directed under Procedure:the relative standard deviation for replicate injections is not more than 2.0%,and the tailing factor is not more than 1.5.
Procedure—
Separately inject equal volumes (about 10µL)of the Standard preparationand the Assay preparationinto the chromatograph,record the chromatograms,and measure the responses for the major peaks.Calculate the quantity,in µg of cefoperazone per mg,of the Cefoperazone Sodium taken by the formula:
1000(C/M)(rU/rS),
in which Cis the concentration,in mg of cefoperazone (C25H27N9O8S2)per mL,of the Standard preparation,Mis the concentration,in mg per mL,of the Assay preparationbased on the weight of Cefoperazone Sodium taken and the extent of dilution,and rUand rSare the peak responses from the Assay preparationand the Standard preparation,respectively.
Auxiliary Information—
Staff Liaison:William W.Wright,Ph.D.,Scientific Fellow
Expert Committee:(PA7)Pharmaceutical Analysis 7
USP28–NF23Page 385
Phone Number:1-301-816-8335