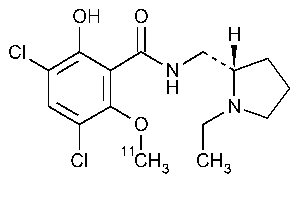
Raclopride C11Injection
»Raclopride C11Injection is a sterile solution,suitable for intravenous administration,of raclopride,in which a portion of the molecules are labeled at the O-methyl position with radioactive 11C.It contains not less than 90.0percent and not more than 110percent of the labeled amount of 11Cexpressed in MBq (or mCi)at the time indicated in the labeling.Its specific activity is not less than 18.5Gbq (500mCi)per µmol.It may contain suitable buffers.
Specific activity—
Mobile phase
and Standard solution—Prepare as directed in the test for Chemical purity.
Chromatographic system—
Proceed as directed in the test for Radiochemical purity.
Procedure—
Calculate the specific activity,in GBq (or mCi)per µmol,of Injection by the formula:
3.47(CrPr)/C,
in which Cris the radioactivity content,in MBq (or mCi)per mL,as determined in the Assay for radioactivity,Pris the radiochemical purity (in %),as determined in the test for Radiochemical purity,and Cis the concentration (in µg per mL)of raclopride in the Injection,as determined in the test for Chemical purity.
Packaging and storage—
Preserve in single-dose or in multiple-dose containers that are adequately shielded.
Labeling—
Label it to include the following,in addition to the information specified for Labelingunder Injections á1ñ:the time and date of calibration;the amount of 11Cas [O-methyl-11C]raclopride,expressed as total megabecquerels (or millicuries);the specific activity,expressed as megabecquerels (or millicuries)per µmol;and the concentration,expressed as megabecquerels (or millicuries)per mL,at the date and time of calibration;the expiration date and time;the lot or batch number;the name and quantity of any added preservative or stabilizer;and the statements,“Caution—Radioactive Material”and “Do not use if cloudy or if it contains particulate matter.”The labeling indicates that in making dosage calculations correction is to be made for radioactive decay,and states that the radioactive half-life of 11Cis 20minutes.
Radionuclide identification á821ñ—
Its gamma-ray spectrum is identical to that of a specimen of 11Cin that it exhibits a positron annihilation peak at 0.511MeVand possibly a sum peak of 1.022MeV,dependent upon geometry and detector efficiency.
Bacterial endotoxins á85ñ—
It contains not more than 175/VUSP Endotoxin Unit per mL,in which Vis the maximum recommended total dose,in mL,at the expiration date or time.
pHá791ñ:
between 4.5and 7.
Radionuclidic purity á821ñ—
Using a multichannel analyzer,count all radioactivity from 40to 2500keVto determine the absence of radiation,other than at 0.511MeVand 1.022MeV,over a period of 4hours.Determine the half-life (20minutes)by a suitable detector system.
Chemical purity—
Mobile phase—
Add 840µLof phosphoric acid to 500mLof deionized distilled water in a 1000-mLvolumetric flask.Add 270mLof acetonitrile,dilute with deionized distilled water to volume,filter,and degas.Make adjustments if necessary (see System Suitabilityunder Chromatography á621ñ).
Standard solution—
Dissolve an accurately weighed quantity of raclopride (as the tartrate salt)in water to obtain a solution having a known concentration of about 1mg of raclopride per mL.Dilute a portion of this solution quantitatively with Mobile phaseto obtain a solution having a known concentration of about 10µg of raclopride per mL.
Test solution—
Prepare a solution by quantitatively diluting an accurately measured volume of Injection,equivalent to about 37MBq (1mCi)of radioactivity,with 10parts of Mobile phase,and mix.
Chromatographic system
(see Chromatography á621ñ)—The liquid chromatograph is equipped with a 254-nm detector and a 3.9-mm ×30-cm column that contains packing L9.The flow rate is about 2mLper minute.Chromatograph the Standard solution,and record the peak responses as directed for Procedure:the relative retention times are about 0.75for O-desmethylraclopride and 1.0for raclopride,the resolution,R,between acetate and carbonate is not less than 1.5,the column efficiency determined from the analyte peak is not less than 85theoretical plates,and the relative standard deviation for replicate injections is not more than 10.0%.
Procedure—
Separately inject equal volumes (about 20µL)of the Standard solutionand the Test solutioninto the chromatograph,record the chromatograms,and measure the areas of the responses for the raclopride peaks.Calculate the concentration,in µg per mL,of raclopride (C15H20Cl2N2O3)in the portion of Injection taken by the formula:
C(rU/rS),
in which Cis the concentration,in µg per mL,of raclopride in the Standard solution,and rUand rSare the raclopride peak responses obtained from the Test solutionand the Standard solution,respectively.In the chromatogram of the Test solution,the area of the peak with a retention time of about 6minutes (raclopride)is not less than 98%of the total area of all peaks.
Radiochemical purity—
Mobile phase
and Standard solution—Prepare as directed in the test for Chemical purity.
Chromatographic system—
Proceed as directed in the test for Chemical purity,except that the liquid chromatograph is also equipped with a suitable collimated radiation detector (see Radioactivity á821ñ).
Procedure—
Inject about 20µLof the Injection into the chromatograph,record the chromatogram,and measure the areas of the responses for the major peaks.The radioactivity under the main peak is not less than 95%of the total area of all peaks observed,and its retention time is within 10%of that obtained for the Standard solution,similarly chromatographed.
Other requirements—
It meets the requirements under Injections á1ñ,except that the Injection may be distributed or dispensed prior to completion of the test for Sterility,the latter test being started on the day following final manufacture,and except that it is not subject to the recommendation on Volume in Container.
Assay for radioactivity á821ñ—
Using a suitable counting assembly (see Selection of a Counting Assemblyunder Radioactivity á821ñ),determine the radioactivity,in MBq (or mCi)per mL,of Injection by use of a calibrated system.
Auxiliary Information—
Staff Liaison:Andrzej Wilk,Ph.D.,Senior Scientific Associate
Expert Committee:(RMI)Radiopharmaceuticals and Medical Imaging Agents
USP28–NF23Page 352
Phone Number:1-301-816-8305