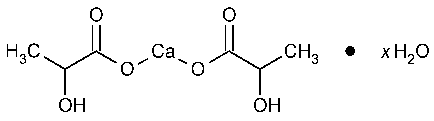
Calcium Lactate
Propanoic acid,2-hydroxy-,calcium salt (2:1),hydrate.
Calcium lactate (1:2)hydrate [41372-22-9].
Calcium lactate (1:2)pentahydrate. 308.30 [5743-47-5].
Anhydrous [814-80-2].
»Calcium Lactate contains not less than 98.0percent and not more than 101.0percent of C6H10CaO6,calculated on the dried basis.
Packaging and storage—
Preserve in tight containers.
Labeling—
The label indicates whether it is the dried form or is hydrous;if the latter,the label indicates the degree of hydration.Where the quantity of Calcium Lactate is indicated in the labeling of any preparation containing Calcium Lactate,this shall be understood to be in terms of calcium lactate pentahydrate (C6H10CaO6·5H2O).
Identification—
A:
Asolution (1in 20)responds to the tests for Calcium á191ñ.
B:
To 10mg add 1mLof sulfuric acid,and heat for 2minutes in a water bath maintained at a temperature of 85 .Cool the solution to room temperature,add about 10mg of 4-phenylphenol crystals,swirl,and allow to stand for about 20minutes:a violet color develops that deepens with the passage of time.
.Cool the solution to room temperature,add about 10mg of 4-phenylphenol crystals,swirl,and allow to stand for about 20minutes:a violet color develops that deepens with the passage of time.
Acidity—
Titrate 20mLof a solution (1in 20)with 0.10Nsodium hydroxide,using phenolphthalein TSas the indicator:not more than 0.50mLis required for neutralization (0.45%as lactic acid).
Loss on drying á731ñ—
Distribute a 1-to 2-g portion evenly in a suitable weighing dish to a depth of not more than 3mm,and dry at 120 for 4hours:the pentahydrate loses between 22.0%and 27.0%of its weight;the trihydrate loses between 15.0%and 20.0%of its weight;the monohydrate loses between 5.0%and 8.0%of its weight;and the dried form loses not more than 3.0%of its weight.
for 4hours:the pentahydrate loses between 22.0%and 27.0%of its weight;the trihydrate loses between 15.0%and 20.0%of its weight;the monohydrate loses between 5.0%and 8.0%of its weight;and the dried form loses not more than 3.0%of its weight.
Volatile fatty acid—
Stir about 500mg with 1mLof sulfuric acid,and warm:the mixture does not emit an odor of volatile fatty acid.
Heavy metals á231ñ—
Dissolve 1g in 2.5mLof 1Nacetic acid,and dilute with water to 25mL:the limit is 0.002%.
Limit of magnesium and alkali salts—
Mix 1.0g with 40mLof water,carefully add 1mLof hydrochloric acid,and heat the solution to boiling.Proceed as directed in the test for Magnesium and alkali saltsunder Calcium Carbonate,beginning with “Rapidly add 40mLof oxalic acid TS”:the weight of the residue does not exceed 5.0mg (1.0%).
Organic volatile impurities,Method Iá467ñ:
meets the requirements.
Assay—
Transfer an accurately weighed amount of Calcium Lactate,equivalent to about 350mg of C6H10CaO6,to a suitable container,and dissolve in a mixture of water and 3Nhydrochloric acid (150:2).While stirring,preferably with a magnetic stirrer,add about 30mLof 0.05Medetate disodium VSfrom a 50-mLburet.Add 15mLof 1Nsodium hydroxide and 300mg of hydroxy naphthol blue,and continue the titration to a blue endpoint.Each mLof 0.05Medetate disodium is equivalent to 10.91mg of C6H10CaO6.
Auxiliary Information—
Staff Liaison:Lawrence Evans,III,Ph.D.,Scientist
Expert Committee:(DSN)Dietary Supplements:Non-Botanicals
USP28–NF23Page 329
Pharmacopeial Forum:Volume No.27(6)Page 3259
Phone Number:1-301-816-8389